Role of Aromatase Inhibitor to Enhance Ovulation in Poor Responder during Induction with Short Antagonist Protocol in Cases of Intracytoplasmic Sperm Injection
Author'(s): Reham El Khateeb*
Minia University Campus, Minia Govern rate, Egypt.
*Correspondence:
Reham El Khateeb, Minia University Campus, Minia Govern rate, Egypt, Tel: +201000222994; E-mail: rehamelkhateeb78@yahoo.com.
Received: 04 January 2018 Accepted: 29 January 2018
Citation: Reham El Khateeb. Role of Aromatase Inhibitor to Enhance Ovulation in Poor Responder during Induction with Short Antagonist Protocol in Cases of Intracytoplasmic Sperm Injection. Gynecol Reprod Health. 2018; 2(1): 1-4.
Abstract
Objective: Study the effect of aromataze inhibitor with short antagonist protocol to enhance ovulation response in patients expected to be poor responder undergoing ICSI.
Methodology: A prospective clinical trial included all patients expected to have poor ovulation response during ovarian stimulation for ICSI. Conducted in Maternity Hospital IVF unite and two tertiary referral infertility private clinics associated with the reproductive sciences division. 50 patients were enrolled in the study divided randomly into two groups. Study group patients were offered letrozole, 2.5 mg/day from day 1-5 of the menstrual cycle with HMG300 IU/day starting on first day of the cycle with follow up by Trans vaginal ultrasound (TVUS) when at least three follicles reach 14mm diameter, GnRh antagonist given 0.25 mg SC injection daily till the day of hCG injection. HCG (10,000 IU) was given when at least three leading follicles were 18mm followed by ovum pick up. Control group offered the same management without letrozole.
Main Outcome Measures: Primary outcome measure: Number of follicles reaches more than 18mm and number of metaphase II oocytes. Secondary outcome measure: clinical pregnancy rate.
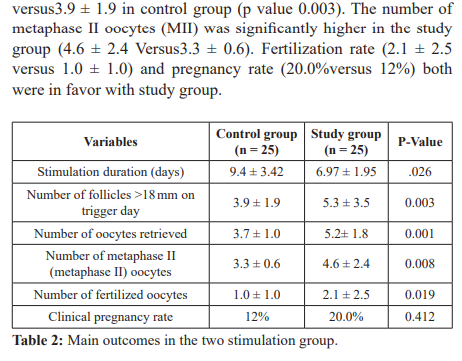
Results: Improved response to HMG stimulation with letrozole co-treatment was evidenced by significant number of follicles measuring more than 18mm (5.3 ± 3.5.in the study group versus 3.9 ± 1.9 in control group (p value 0.003). The number of metaphase II oocytes was significantly higher in the study group (4.6 ± 2.4 Versus3.3 ± 0.6). During letrozole co treatment clinical pregnancy was achieved in (20%) of cases.
Conclusion: We demonstrated a good benefit of aromatase inhibitors for improving ovarian response to HMG in short antagonist protocol in patients expected to be poor responders.
Keywords
Introduction
Although there is no standard definition for poor ovarian response, the European Society for Human Reproduction and Embryology has proved that at least two of the three features must be present in poor ovarian response: advanced maternal age or any other risk factor for poor ovarian response; previous poor ovarian response; or an abnormal ovarian reserve test (Bologna Criteria) [1].
Poor ovarian response to ovarian hyperstimulation is one of the biggest challenges and frustrating for both patients and clinician. Although many stimulation protocols have been used to improve outcomes in poor ovarian responders (PORs), there is a controversy which protocol is the most effective [2].
Despite the prediction of low response to ovarian stimulation remains problem different tests have shown variable success, including day 3 FSH, clomiphene citrate (CC) challenge test, and inhibin concentrations, antral follicular count and serum AMH also can predict low response to ovarian stimulation [3].
Several strategies have been proposed to improve outcome in low responders. The use of GnRHant is a commonly used protocol for pituitary downregulation for improving ovarian stimulation response in poor responders. Mechanism of action it leads to immediate, rapid gonadotropin suppression by competitively blocking GnRH receptors in the anterior pituitary gland, so prevent endogenous premature release of LH and FSH. Several RCTs did not show any significant difference in clinical pregnancy rate or number of oocytes retrieved with the use of GnRHant [4-20].
Aromatase is enzyme that inhibits the conversion of the androgens, androstenedione and testosterone, to estrone (E1) and E2. The development of more specific and potent aromatase inhibitors, including letrozole is orally active and is well tolerated with no significant side effects [21].
Methodology
The study is a prospective clinical trial. Approved by Department of Obstetrics and Gynecology faculty of medicine (Number: MUH20168) conducted in Maternity hospital IVF unite Minia University and two tertiary referral infertility private clinics associated with the reproductive sciences division. The study was conducted during the period from August 2016 to August 2017, all study details were explained to patients and informed consent was obtained before inclusion in the study. Enrolled patients with eligible criteria were randomized into two groups using simple randomization by sealed opaque envelops contain serial computer generated numbers. Control group offered induction with HMG 300 IU (Merional ®, IBSA, Turkey) daily start at first day of menses with follow up by trans vaginal ultrasound (TVUS) when at least three follicles reach 14mm diameter GnRh antagonist given 0.25 mg ganirelix SC (Orgalutran; NV Organon, Oss, the Netherlands) with continuous follow up. Ovarian follicular development was monitored by transvaginal ultrasonography and serum levels of E2 a HCG 10,000 IU (Profasi, Serono, Oakville, Ontario, Canada) was given to trigger ovulation when three leading follicles reached a diameter 18mcm. The hCG administration was followed by ovum pick up under transvaginal ultrasound guide. Fertilization of the aspirated oocytes was carried out in vitro, by ICSI. Embryos were examined for the number and regularity of blastomeres and the degree of embryonic fragmentation, and graded according to Cummins's criteria. All highest-quality embryos (including grade 1 and grade 2) were transferred on the day 5. Pregnancy was diagnosed by quantitative assay of serum B HCG 2 weeks after embryo transfer and clinical pregnancy was confirmed by identification of a positive fetal heart beat at 6-7 weeks’ gestation by transvaginal ultrasonography. Study group received the same management plus letrozole (Femara; Novartis, East Hanover, NJ) was given at a dose of 2.5 mg from day 1 to 5 of the menstrual cycle with same protocol and follow up. Both groups were compared as regard primary and secondary outcomes.
Inclusion criteria
Patients indicated for ICSI due to (Ovarian factor, tubal factor and unexplained infertility.) and expected to be poor responder, had to meet at least two of the following criteria (age over 37 years; a history of ovarian surgery; antral follicle count of less than 6 on menstrual cycle day 2-3; and basal serum FSH concentration between 10 and 19 IU/l, serum AMH (0.5 -1 IU).
Exclusion criteria
High responder, endometriosis, male factor, uterine factors, ovarian mass or cyst, documented ovarian failure.
Statistical analysis
Primary outcome of the study was number of follicles reach more than 18mm, number of metaphase II oocytes if more, Secondary outcome was pregnancy rate. Data were presented as the mean + standard deviation (SD) in text and tables. Data were analyses by Student's t-test, Mann–Whitney U test and chi-squared test where appropriate. The one-way analysis of variance method was used for the comparison of hormone concentrations at different time- points. The Mann–Whitney U test was used for the variables of non-normal distribution. The significance was accepted for P < 0.05. All data were analyses using the Statistical Package for the Social Sciences for Windows (SPSS, Version 16.0).
Results
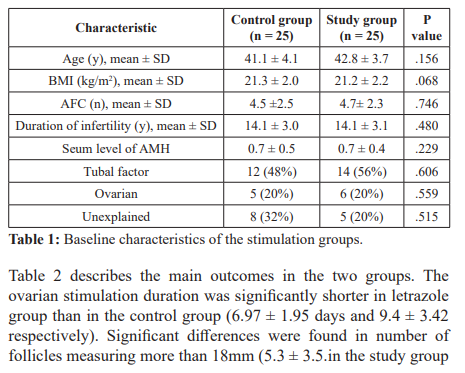
58 patients, characterized as poor responders according to the Bologna criteria. Eight patients were excluded due to treatment discontinuation because of poor stimulation response. Baseline characteristics of the patients in the two ovarian stimulation groups are presented in table 1. There were no differences in age, BMI, AFC, duration of infertility and serum level of AMH. The main indications for ICSI treatment were noted to be tubal factor, ovarian factor and unexplained. There were no differences in these infertility indications among the two stimulation groups.
Discussion
Letrozole succeeded in inducing ovulation in an ovulatory woman with polycystic ovary syndrome (PCOS) [23-25] and enhancing ovulation in ovulatory women. Moreover, that when letrozole was used with FSH, a significant reduction occurred in FSH dose needed for controlled ovarian hyperstimulation [26,27]. Mitwally et al. demonstrated the benefit of letrozole in improving ovarian response to FSH stimulation in poor responders. The improved response is clearly shown by the significantly higher number of mature follicles and significantly lower doses of drugs used for induction [3].
In the current study we demonstrated the benefit of letrozole co treatment during ovarian hyperstimulation with short antagonist protocol in patients undergoing ICSI. This may be attributed to increase follicular sensitivity to endogenous FSH as a result of a temporary accumulation of intraovarian androgens, as the conversion of the androgen substrates to estrogen is blocked. Although there is no standard protocol for poor responder but several studies have proved better ovarian response with antagonist protocol. However the previous two studies started stimulation in the luteal phase [2,28].
In current study Letrozole co treatment was associated with a significantly higher number mature follicles and higher pregnancy rate. Letrozole decreases E2 in COH cycles that may be beneficial in avoiding the possible undesired effects of the supraphysiologic levels of E2 associated with ovarian hyperstimulation. Markedly elevated E levels have been reported to have harmful effects on the embryo and endometrium that endanger the chance of implantation and pregnancy [29,30].
This preliminary results of current study includes a small number of poor responder patients treated short antagonist protocol with lerozole however, our objective was to confirm the idea of adjuvant letrozole treatment can improve ovarian response in this selected group. We know that ICSI trail is so expensive. In low resources
countries insurance did not cover the cost of treatment trial. Our aim is to improve patient response for stimulation, decrease HMG dose and avoid cycle cancellation that can markedly reduce stress and cost of ICSI trial. We encourage controlled prospective randomized trial in the future to confirm the benefit of letrozole co treatment in short antagonist protocol in poor responder.
References
- Ferraretti AP, La Marca A, Fauser BC, et ESHRE consensus on the definition of ‘poor response'to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum. Reprod. 2011; 26: 1616-1624.
- Li-Hong Wei, Wen-Hong Ma, Ni Tang, et al. Luteal-phase ovarian stimulation is a feasible method for poor ovarian responders undergoing in vitro fertilization/intracytoplasmic sperm injection-embryo transfer treatment compared to a GnRH antagonist protocol: A retrospective study. Taiwan J Obstet Gynecol. 2016; 55: 50-54.
- Mitwally M, Casper Aromatase inhibition improves ovarian response to follicle stimulating hormone in poor responders. Fertil Steril. 2002; 77: 776-780.
- Cheung LP, Lam PM, Lok IH, et GnRH antagonist versus long GnRH agonist protocol in poor responders undergoing IVF: A randomized controlled trial. Hum Reprod. 2005; 20: 616-621.
- Demirol A, Gurgan T. Comparison of microdose flare-up and antagonist multiple-dose protocols for poor-responder patients: A randomized Fertil Steril. 2009; 92: 481-485.
- De Placido G, Mollo A, Clarizia R, et al. Gonadotropin- releasing hormone (GnRH) antagonist plus recombinant luteinizing hormone vs. A standard GnRH agonist short protocol in patients at risk for poor ovarian response. Fertil Steril. 2006; 85: 247-250.
- Devesa M, Martínez F, Coroleu B, et al. Poor prognosis for ovarian response to stimulation: Results of a randomised trial comparing the flare-up GnRH agonist protocol vs. The antagonist protocol. Gynecol Endocrinol. 2010; 26: 509-515.
- DiLuigi AJ, Engmann L, Schmidt DW, et al. A randomized trial of microdose leuprolide acetate protocol versus luteal phase ganirelix protocol in predicted poor responders. Fertil Steril. 2011; 95: 2531-2533.
- Kahraman K, Berker B, Atabekoglu CS, et al. Microdose gonadotropin-releasing hormone agonist flare-up protocol versus multiple dose gonadotropin-releasing hormone antagonist protocol in poor responders undergoing intracytoplasmic sperm injection-embryo transfer Fertil Steril. 2009; 91: 2437-2444.
- Karimzadeh MA, Mashayekhy M, Mohammadian F, et Comparison of mild and microdose GnRH agonist flare protocols on IVF outcome in poor responders. Arch Gynecol Obstet. 2011; 283: 1159-1164.
- Liu XQ, Wang WF, Tan DX. Clinical outcomes of gonadotropin-releasing hormone antagonist used in poor ovarian responders. Wei Chung Yi Xue. 2009; 4: 657-659.
- Malmusi S, La Marca A, Giulini S, et al. Comparison of a gonadotropin-releasing hormone (GnRH) antagonist and GnRH agonist flare-up regimen in poor responders undergoing ovarian stimulation. Fertil Steril. 2005; 84: 402-406.
- Marci R, Caserta D, Dolo V, et al. GnRH antagonist in IVF poor-responder patients: Results of a randomized Reprod Biomed Online. 2005; 11: 189-193.
- Martínez F, Coroleu B, Marqués L, et Comparison of ‘short protocol’ versus ‘antagonists’ with or without clomiphene citrate for stimulation in IVF of patients with ‘low response. Rev Iberoam Fert Rep Hum. 2003; 20: 355-360.
- Prapas Y, Petousis S, Dagklis T, et al. GnRH antagonist versus long GnRH agonist protocol in poor IVF responders: A randomized clinical trial. Eur J Obstet Gynecol Reprod Biol. 2013; 166: 43-46.
- Schmidt DW, Bremner T, Orris JJ, et al. A randomized prospective study of microdose leuprolide versus ganirelix in in vitro fertilization cycles for poor responders. Fertil Steril. 2005; 83: 1568-1571.
- Sunkara SK, Coomarasamy A, Faris R, et al. Long gonadotropin-releasing hormone agonist versus short agonist versus antagonist regimens in poor responders undergoing in vitro fertilization: A randomized controlled Fertil Steril. 2014; 101: 147-153.
- Tazegül A, Görkemli H, Ozdemir S, et al. Comparison of multiple dose GnRH antagonist and minidose long agonist protocols in poor responders undergoing in vitro fertilization: A randomized controlled trial. Arch Gynecol Obstet. 2008; 278: 467-472.
- Tian L, Lu Q, Shen H, et Gonadotropin-releasing hormone antagonist plus HMG improve the pregnancy rate of IVF-ET on poor responders. Chin J Clin Obstet Gynecol. 2008; 9: 38- 53.
- Wang B, Sun HX, Hu YL, et al. Application of GnRH- antagonist to IVF-ET for patients with poor ovarian Zhonghua Nan Ke Xue. 2008; 14: 423-426.
- ESHRE Capri Workshop Group Health and fertility in World Health Organization group 2 anovulatory women .Hum Reprod Update. 2012; 18: 586-599.
- Mitwally MFM, Casper The use of an aromatase inhibitor for induction of ovulation in cases of clomiphene citrate failure. In Program and abstracts of the 16th Annual Meeting of the European Society for Human Reproduction and Embryology (ESHRE) Bologna, Italy. 2000.
- Mitwally MFM, Casper RF. Aromatase inhibition: a novel method of ovulation induction in women with polycystic ovarian syndrome. Reprod Technol. 2000; 10: 244-247.
- Mitwally MFM, Casper The aromatase inhibitor, letrozole: a promising alternative for clomiphene citrate for induction of ovulation. In: Program and abstracts of the 56th Annual Meeting of the American Society for Reproductive Medicine (ASRM). 2000.
- Mitwally MFM, Casper Use of an aromatase inhibitor for induction of ovulation in patients with an inadequate response to clomiphene citrate. Fertil Steril. 2001; 75: 305-309.
- Mitwally MFM, Casper The aromatase inhibitor, letrozole, decreases FSH dose required for ovarian superovulation. The 46th Annual Meeting of the Canadian Fertility and Andrology Society. Newfoundland, Canada. 2000.
- Mitwally MFM, Casper RF. Aromatase inhibition decreases FSH dose needed during controlled ovarian hyperstimulation: a controlled prospective trial. Meeting of the Society for Gynecologic Investigation. Toronto, Canada. 2001.
- Kuang Y, Hong Q, Chen Q, et al. Luteal-phase ovarian stimulation is feasible for producing competent oocytes in women undergoing in vitro fertilization/intracytoplasmic sperm injection treatment, with optimal pregnancy outcomes in frozen–thawed embryo transfer cycles. Fertil Steril. 2014; 101: 105-111.
- Tucker KE. Reproductive toxicity of ovulation induction. Semin Reprod Endocrinol. 1999; 14: 345-353.
- Gelety TJ, Buyalos RP. The influence of supraphysiologic estradiol levels on human nidation. J Assist Reprod Genet. 1995; 12: 406-412.