Stress Promoted Nicotine Intake in a Rat Model of Tobacco Smoking
Author'(s):Thuy Tran, Trisha Patel, Treniea Tolliver, Ethan Westbrook and Xiu Liu*
Department of Pathology, University of Mississippi Medical Center, Jackson, MS 39216.
*Correspondence:
Xiu Liu, MD, PhD, Professor, Department of Pathology, University of Mississippi, Medical Center, 2500 North State Street, Jackson, MS 39216, Tel: (601) 984-2875.
Received:11 January 2021;Accepted:05 February 2021
Citation: Tran T, Patel T, Tolliver T, et al. Stress Promoted Nicotine Intake in a Rat Model of Tobacco Smoking. Addict Res. 2021; 5(1): 1-10.
Abstract
Epidemiological documents show an association of tobacco smoking rates and perceived stress levels. This study, using an animal model of nicotine self-administration, investigated effects of stress on nicotine-taking behavior. Sprague-Dawley rats were trained to intravenously self-administer nicotine. Thirty minutes before test sessions, animals were challenged with an intraperitoneal administration of a pharmacological stressor yohimbine. In the low nicotine-taking rats, yohimbine challenge enhanced lever-press responses and thereby nicotine intake. In contrast, no such effect was observed in the high nicotine-taking rats. After yohimbine challenge, nicotine intake in those originally low nicotine-taking rats remained at the heightened level. These findings demonstrate that exposure to stress facilitates nicotine self-administration in the rats previously consuming less nicotine and makes them to become high nicotine-taking subjects. The results of this study suggest that stressful life events may be effective in increasing tobacco smoking in light to moderate smokers and therefore increase the prevalence of nicotine dependence. As such, reducing stress levels in daily life may prove to be an effective approach to the prevention of nicotine addiction.
Keywords
Introduction
Clinical evidence indicates that stress contributes to initiation, maintenance and progression of tobacco smoking [1,2]. For instance, college students increased smoking during periods of high stress such as the time leading up to final examination [3,4]. In a sample of 2,961 adolescents, emotional distress in 10th grade was associated with increased smoking in these individuals once they were in 12th grade [5]. Smokers reportedly have more stressful situations and negative life events than do nonsmokers [2,6-8] and stress increases craving for tobacco [9-12]. Subjective accounts indicate that smokers use tobacco smoking to reduce their stress levels [13,14] and expect smoking to decrease stress-induced negative affect [15,16]. Indeed, numerous laboratory studies have found smoking produces reduction in negative affect associated with stress exposure [10,15,17,18]. Animal studies have shown that nicotine, the main addictive component of tobacco, attenuates stress-induced negative affect [19-21] and reduces stress-induced immobilization and changes in corticomesolimbic dopamine levels [22-24]. Taken together, these clinical data indicate stress as a facilitator of tobacco smoking and nicotine dependence.
In animal research, it has been evidenced that acute stress exposure effectively reinstates nicotine-seeking behavior in procedures modeling human smoking relapse [25-29]. To date, however, it remains unknown whether acute stress exposure influences nicotine intake in animal models of nicotine self-administration. The present study addressed this issue by challenging animals with pharmacological stressor, yohimbine, after establishment of stable nicotine intake. Yohimbine is a α2 adrenergic receptor antagonist. It increases activity of noradrenergic systems including neural structures implicated in stress response [30-33] and produces anxiety- and stress-like states in humans and laboratory animals [34-39]. It has been increasingly used as a pharmacological stressor, especially in the field of drug addiction research [40-48]. Importantly, yohimbine-induced reinstatement of drug-seeking behavior in animal models of drug relapse appears to be more robust than that elicited by foot shock stress [49-52]. Therefore, this study employed yohimbine as an anxiogenic and stress-inducing agent to determine its effect on nicotine self-administration behavior.
Moreover, there are substantial individual differences in smoking behavior in humans and in nicotine self-administration in animals. Studies on individual differences in drug self-administration have usually focused on the effects of pre-existing behavioral profiles on drug intake. For instance, levels of loco motor response to novelty [53-57], anxiety-like behavior in the elevated plus-maze [58], and consumption of sucrose [59,60] have been found to predict the propensity to self-administer psychostimulants including nicotine. However, in the operant nicotine self-administration literature animals that self-administer lower levels of nicotine are often excluded from experiments [61-64]. Consequently, no attempt has been made to examine the effect of smoking-predisposing factors such as stress in these low nicotine-taking subjects. Therefore, the second goal of this study was to compare the effects of stress on nicotine self-administration behavior in rats that differed in their nicotine intake prior to acute yohimbine challenge.
To get the most robust operant behavior for nicotine intake, this study used a nicotine unit dose of 0.03 mg/kg/infusion because it was the peak dose on dose-response curve for nicotine self- administration [65-67]. After establishment of stable nicotine self- administration, animals were designated as the low nicotine-taking (LNT) that self-administered ≤ 6 infusions/session and the high nicotine-taking (HNT) rats that self-administered > 6 infusions/ session. Such a cut-off criterion was made based on two reasons. First, in adult smokers, it has been suggested that 5 cigarettes/day would be a reasonable threshold for establishing nicotine addiction[68] and that each cigarette has been estimated to deliver as much as 0.03 mg/kg to smokers [69]. Accordingly, six infusions/session at a unit dose of 0.03 mg/kg/infusion (free base) were comparable to the threshold for nicotine addiction. Therefore, the LNT rats could be taken as an equivalent to “social” or “light” human smokers, whereas the HNT rats could serve as the subjects with heavy smoking or addicted to nicotine. Second, as mentioned above, rats showing a lower nicotine intake have been usually excluded from experiments in order for the self-administration paradigm to serve as a valid model of nicotine addiction. For example, although the specific cut-off criteria reported in literature varied, rats self- administering ≤ 6 infusions/session at 0.03 mg/kg/infusion of nicotine are always excluded from experiments [61-64].
Methods
Subjects
Male Sprague-Dawley rats (Charles River, Portage, MI, USA) weighing 200-225 g upon arrival were used. Animals were individually housed in a humidity- and temperature-controlled (21-22oC) colony room on a reversed light/dark cycle (lights on 20:00; off 8:00) with unlimited access to water. After one week habituation, rats were placed on a food-restriction (20 g chow/day) regimen throughout the experiments. Training and experimental sessions were conducted during the dark phase at the same time each day (10:00-16:00). All experimental procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved (#1183) by the University of Mississippi Medical Center Institutional Animal Care and Use Committee.
Self-administration apparatus
Experimental sessions were conducted in the standard operant conditioning chambers located inside sound-attenuating, ventilated cubicles (Med Associates, St. Albans, VT, USA). The chambers were equipped with two retractable response levers on one side panel and with a 28-V white light above each lever as well as a red house light on the top of the chambers. Between the two levers was a food pellet trough. Intravenous nicotine injections were delivered by a drug delivery system with a syringe pump (Med Associates, model PHM100-10 rpm). Experimental events and data collection were automatically controlled by an interfaced computer and software (Med Associetes, Med-PC® IV).
Food training
To facilitate learning of operant responding for nicotine self- administration (see below), rats began food training sessions on the day following the initiation of food-restriction regimen. Responses on the active lever were rewarded with delivery of a food pellet (45 mg). Sessions lasted 1-h with a maximum delivery of 45 food pellets on a fixed-ratio (FR) 1 schedule. Once the rats learned responding and earned 45 pellets total in a session, the reinforcement schedule was increased to FR5. Successful food training was achieved once rats earned in a single session the total 45 food pellets on the FR5 schedule. Food training was typically completed within 2-5 sessions.
Surgery
After food training, the rats were anesthetized with an isoflurane- oxygen mixture (1-3% isoflurane) and implanted with jugular catheters. Catheters were constructed using a 15 cm piece of Silastic tubing (0.31 mm ID and 0.63 mm OD, Dow Corning Corporation, Midland, MI, USA) attached to a 22-gauge stainless-steel guide cannula. The latter was bent and molded onto a durable polyester mesh (Plastics One Inc., Roanoke, VA, USA) with dental cement and became the catheter base. Through an incision on the rat back, the base was anchored underneath the skin at the level of scapulae and the catheter passed subcutaneously to the ventral lower neck region and inserted into the right jugular vein (3.5 cm). Animals were allowed at least 7 days to recover from surgery. During the recovery period, the catheters were flushed daily with 0.1 ml of sterile saline containing heparin (30 U/ml) and time tin (66.7 mg/ ml) to maintain catheter patency and prevent infection. Thereafter, the catheters were flushed with the heparinized saline before and after the experimental sessions throughout the experiments.
Nicotine self-administration
After recovery from surgery, rats were trained to intravenously self- administer nicotine (0.03 mg/kg/infusion, free base). In the training sessions, animals were placed in the experimental chambers and connected to a drug delivery system. The daily 1-h sessions were initiated by extension of the two levers and illumination of the red house light. Once the rats reached the FR requirement on the active lever, an infusion of nicotine was dispensed by the drug delivery system in a volume of 0.1 ml in approximately 1 s depending on rat body weights. Each nicotine infusion was signaled with a presentation of a stimulus (5 s tone/20 s lever light on). The stimulus also signaled a 20 s timeout period, during which time responses were recorded but not reinforced. Responses on the inactivate lever had no consequence. An FR1 schedule was used for days 1-5, an FR2 for days 6-8 and an FR5 for remainder of the experiments. To test effect of pharmacological stress on nicotine self-administration during the maintenance phase, all rats received 20 daily training sessions. This procedure facilitated responding for nicotine self-administration before the start of drug treatment.
To control for possible nonspecific arousal and/or motor-activating effect of yohimbine stress, a separate set of rats was trained under similar conditions, except that saline rather than nicotine infusions were available.
Effect of pharmacological stress on nicotine intake
Based on the number of nicotine infusions/session averaged across the final 5 sessions of the 20 daily training sessions, animals were divided into two groups. Rats that earned 1-6 nicotine infusions/ session were designated as the LNT rats (n=12), whereas rats taking more than 6 nicotine infusions/session as the HNT rats (n=11). Following this, the yohimbine test sessions began. Thirty min before tests, yohimbine (0, 0.5, 1, 2 mg/kg) was intraperitoneally administered using a within-subject, Latin Square design in both the LNT and the HNT rats. The test sessions were performed under conditions exactly the same as described above and scheduled on every other day, i.e., sessions 21, 23, 25 27. Rats still received nicotine self-administration sessions without yohimbine pretreatment between the test sessions. The control group of animals that received saline rather than nicotine infusions (n=11) was subjected to the same yohimbine treatment arrangement.
Statistical analyses
Data were presented as the mean (± SEM) number of lever responses and nicotine infusions. A two-way ANOVA was used to analyze lever response data averaged across the final 5 sessions of the training phase with Group (LNT vs. HNT) as the between- subject factor and Lever (active vs. inactive) as the within-subject factor. The number of nicotine infusions in the final 5 training sessions was analyzed using a two-way ANOVA with repeated measures with Group (LNT vs. HNT) as the between-subject factor and Session as the within-subject factor. The data obtained from yohimbine tests were analyzed using one-way ANOVA. Differences among individual means were verified using the Fisher’s PLSD post hoc tests.
Results
Nicotine self-administration in the LNT and HNT rats
In the 20 daily 1-h training sessions, rats of both the LNT and the HNT groups readily acquired nicotine self-administration behavior. Since there was a small percentage of rats in each experiment that showed lower level of nicotine intake, the LNT rats (n=12) for this study were pooled across two experiments.
Correspondingly, the HNT rats (n=11) were also allocated/ matched from these experiments. As shown in Figure 1, there was a significant difference between the LNT and the HNT rats in the number of lever responses and corresponding nicotine infusions. A two-way ANOVA on the lever responses averaged across the final 5 self-administration sessions before yohimbine tests yielded significant effect of Group [F(1,21) = 60.70, p< 0.0001] and Lever [F(1,21) = 142.66, p< 0.0001] as well as significant Group x Lever interaction [F(1,21) = 44.66, p< 0.0001. Furthermore, Fisher’s PLSD post hoc tests revealed significant difference between the LNT and the HNT rats in the number of responses on both the active (p< 0.01) and inactive (p< 0.01) levers. Correspondingly, the LNT rats, compared to their HNT counterparts, earned significantly less nicotine injections per 1-h session, 4.6 ± 1.1 vs. 16.5 ± 1.2 averaged across the final 5 sessions. In both groups of rats, the number of responses on the active lever was significantly (p< 0.01) higher than on the inactive lever, indicating that both the LNT and the HNT rats successfully acquired nicotine self-administration behavior? The LNT and the HNT rats showed similar body weight gain during these experiments with 328

Figure 1: Profiles of nicotine self-administration in the LNT (n=12) and the HNT (n=11) rats. Top panel shows the number of lever responses averaged across the final 5 sessions of the 20 daily 1-h training phase; Bottom panel presents nicotine infusions earned in the final 5 sessions. Data are expressed as mean ± SEM. * p< 0.05, ** p< 0.01 significant difference from the HNT rats.
Effect of yohimbine on nicotine self-administration in the LNT rats
As shown in Figure 2, yohimbine pretreatment significantly increased nicotine self-administration. One-way ANOVA on the number of responses on the active lever revealed a significant effect of Dose [F (3,44) = 3.11, p< 0.05]. Furthermore, Fisher’s PLSD post hoc tests revealed significant difference between 2 mg/ kg (p< 0.01) and 1 mg/kg (p< 0.05) vs. saline control condition. Correspondingly, yohimbine pretreatment dose-dependently increased the number of nicotine injections [F(3,44) = 3.16, p< 0.05]. However, responses on the inactive lever remained low and indistinguishable among different test conditions. Moreover, in the subsequent 5 sessions conducted following the yohimbine test the active lever responses remained at a significantly higher level relative to the pre-test sessions, a level similar to that maintained by the HNT rats. Detailed information is shown in Figure 5.
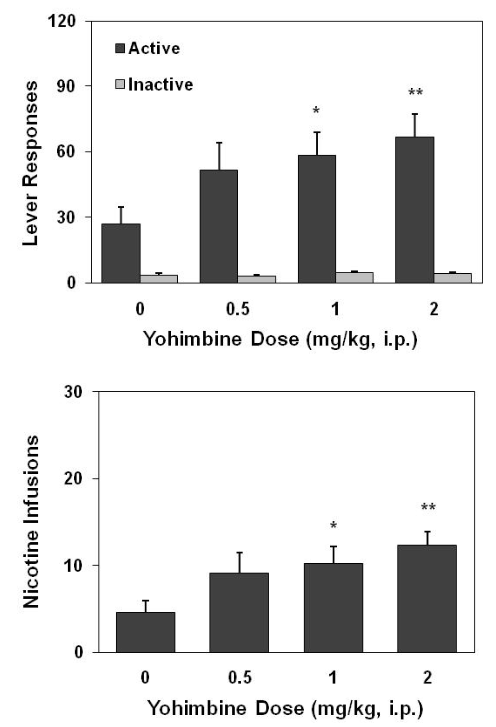
Figure 2: Effect of yohimbine on lever responses (top) and nicotine infusions earned (bottom) in the LNT rats (n=12). Thirty min before test, yohimbine was intraperitoneally administered in a within-subject, Latin Square design on every other day. Data are expressed as mean ± SEM. * p< 0.05, ** p< 0.01 significant difference from saline control (0) condition.
Effect of yohimbine on nicotine self-administration in the HNT rats
Figure 3 shows the lever responses made by the HNT rats after yohimbine pretreatment. A one-way ANOVA on the number of responses on the active lever did not yield a significant effect of Dose [F(3,40) = 0.49, p = 0.69]. Similarly, there was no change observed on the inactive lever responses. Besides, the level of nicotine self-administration in the 5 sessions following yohimbine challenge remained comparable to the pre-test level (Figure 5).

Figure 3: No effect of yohimbine pretreatment on nicotine self- administration behavior in the HNT rats (n=11). Thirty min before test, yohimbine was intraperitoneally administered in a within-subject, Latin Square design on every other day. Data are expressed as mean ± SEM.
Effect of yohimbine in saline-trained rats
During the 20 daily sessions, these rats showed low, but steady, levels of responses on the active lever that delivered presentation of the stimulus with saline rather than nicotine infusions. The mean ± SEM number of responses averaged across the final 5 sessions before yohimbine tests were 15 ± 3 on the active and 4 ± 1 on the inactive levers. One-way ANOVA on the number of responses on the active lever obtained from yohimbine test sessions yielded no significant dose effect [F(3,40) = 1.10, p = 0.36], indicating that yohimbine did not change lever responding in these control rats (Figure 4). Further, after yohimbine tests lever responses in these animals remained at low levels comparable to pre-test sessions (Figure. 5).
Discussion
The current study shows that pharmacological stress induced by acute yohimbine administration significantly increases operant nicotine self-administration in rats that self-administer low levels of nicotine at baseline. Interestingly, under the same testing conditions, yohimbine challenge did not alter nicotine self-administration in rats that self-administered relatively high levels of nicotine at baseline. These results suggest that stress may prompt tobacco smoking in low to moderate smokers, which in turn could increase the prevalence of heavy smoking and nicotine dependence. Moreover, it is recommended that the low nicotine-

Figure 4: No effect of yohimbine pretreatment on lever responses in the rats (n=11) that had never self-administered nicotine. Responses on the active lever resulted in saline infusions and presentations of the stimulus. Data are expressed as mean ± SEM.

Figure 5: Top panel shows the sustained high level of nicotine self- administration behavior after exposure to yohimbine challenge in the LNT rats (N=12). For comparison, active lever responses emitted by the HNT rats (n=11) and the saline control counterparts (n=11) are shown. Bottom panel presents the number of nicotine infusions averaged across the 5 sessions following yohimbine challenge. Data are expressed as mean ± SEM. ** p< 0.01 significant difference from both the pre-yohimbine condition of the HNT rats and the post-yohimbine condition in the LNT rats taking animals may serve as good subjects for investigation on the risk factors precipitating tobacco smoking and the transition to nicotine addiction.
For proper interpretation of these behavioral data, there are two potential confounding factors that need to be addressed. One is the possible nonspecific arousal effect of yohimbine on locomotor activity. The other is that yohimbine might have produced an enhancing effect on the lever responses depending on the level of baseline responses, i.e., facilitating responses when the pre-test responses were low. The latter has been reported in literature. For example, a partial NMDA agonist D-cycloserine was found to decrease nicotine self-administration in rats with low but not high baseline levels of response [70]. To rule out these confounding factors, this study included a control group. For the rats of this control group, active lever responses on a FR5 schedule resulted in delivery of saline rather than nicotine, together with presentation of the stimulus that was used as nicotine cue in the nicotine-trained rats. As shown in figure 5, these rats emitted very low (lower than that maintained by the low nicotine-taking rats) but steady level of responses on the active lever. Pretreatment with yohimbine did not change lever responses in these rats as shown in figure 4. Together with the lack of effect of yohimbine in the rats that had already developed high level of nicotine self-administration as shown in figure 3, these control data negate any nonspecific action of yohimbine. Therefore, the pharmacological stress induced by yohimbine treatment selectively increased lever responses for nicotine self-administration in the LNT rats.
In drug self-administration literature, a lot attention has been paid to the predisposing traits such as response to novelty [53- 57], anxiety levels [58], and consumption of sucrose [59-60]. Differences in these traits have been found to co-vary with levels of self-administration of various drugs of abuse. For instance, rats showing relatively high levels of loco motor response to novel environments acquire nicotine self-administration more readily and have higher breaking points for nicotine under progressive- ratio schedules of reinforcement than do rats with lower loco motor responsiveness to novelty [55]. However, using such pre-existing traits as a drug intake predictor calls for debate because negative observations have been reported. For example, [56] did not find any influence of anxiety level on the preference and consumption of nicotine in mice in a free choice, home cage nicotine-drinking procedure. The degree of stress reactivity did not alter operant nicotine self-administration in a nose-poke procedure in mice selectively bred for different stress response [71]. Interestingly, in the latter study [71], reinstatement of nicotine-seeking behavior induced by foot shock stress was observed only in the mice with highest stress reactivity. That finding was in line with human studies showing that acute psychological stress usually increased smoking desire [7,72-75] but not the amount of smoking if allowed to smoke [75]. The present study for the first time used the level of nicotine intake as a preexisting condition to test how acute stress changes nicotine self-administration in the maintenance phase. The results suggest that the level of nicotine consumption may present a new measurement that can be used to predict how stress changes drug intake. Obviously, more work on the issue will be warranted.
Acute pharmacological stress immediately increased nicotine self-administration in the LNT rats to a level comparable to that maintained by the HNT rats. As such, stress seemed to eliminate the pre-existing difference in nicotine intake between the LNT and the HNT rats. This finding is in line with previous reports showing that systemic administration of corticosterone can eliminate difference in self-administration of cocaine and amphetamine between rats that differed in response to novelty [76,77]. Taken together, these studies and the current data suggest that stress acted to equalize drug consumption across subjects that initially self-administered different amount of drugs of abuse. Even more significant is the find that after acute yohimbine challenge the LNT rats showed sustained high level of nicotine intake similar to the of the HNT counterparts. That means that exposure to stress has transformed the LNT into HNT rats. If the LNT rats are comparable to social smokers or light smoking whereas the HNT rats represent the heavy smokers, it is conceivable that exposure of stressful life events may prompt smoking in light smokers and thereby lead to heavy tobacco smoking. Therefore, stress may serve as an important risk factor for the transition from social smoking to heavy smokers or nicotine addiction.
One of the most prominent effects of stress is the activity of the hypothalamic-pituitary-adrenal (HPA) axis. The HPA axis has been implicated in the process of drug addiction including tobacco smoking [78-81]. Nicotine has been shown to produce changes in the HPA axis functions. Acute nicotine self-administration activates the HPA axis [82-85]. Effects of nicotine taken by operant self-administration procedures seem to be complex, from modest increase to suppression of the HPA functions [86-90]. It is postulated that the HNT rats compared to the LNT counterparts may have a blunted HPA response to stress, which might underlie the inability of yohimbine to facilitate nicotine intake in the HNT rats. In contrast, yohimbine may have substantially activated the HPA axis and thereby increased nicotine self-administration. Unfortunately, there was a limitation in the experimental design, which lacked measurement of HPA hormones in response to the yohimbine challenge. The role of HPA response to stress in nicotine intake between the LNT and HNT subjects deserves future investigation.
Increasing evidence suggests that both genetic and environmental factors, especially interactions between these factors contribute to individual vulnerability to drugs of abuse [91-94]. The difference in nicotine consumption between the LNT and HNT rats may reside in genetic background of these animals. Such different genetic backgrounds may render the subjects to distinct profiles in terms of stress sensitization of the reinforcing properties of nicotine, a phenomenon reported from animal research [83,95-98] and indicated by studies in human smokers [99,100]. In addition, our data showing a facilitating effect of acute pharmacological stress on nicotine self-administration only in the LNT rats may in fact represent an interaction between the genetic and environmental factors.
Conclusions
The present study, using a rat model of nicotine self- administration, examined effect of acute pharmacological stress on nicotine intake with an emphasis on the comparison between rats that initially showed significantly different levels of nicotine self-administration. The results demonstrated that yohimbine challenge increased nicotine intake in the LNT rats but did not alter nicotine self-administration in the HNT rats. Importantly, after exposure to the yohimbine-induced stress, the heightened nicotine self-administration was sustained at a level comparable to that maintained by the HNT rats. These findings indicate that stress exposure transforms the LNT rats into the HNT subjects, suggesting that stressful life events may be particularly effective in prompting tobacco smoking in light to moderate smokers and therefore contribute to the high prevalence of heavy smokers and nicotine addiction. This is in line with previous reports that stressful life events contribute to the development of drug use and addiction [98,101-104]. As such, it is proposed that reducing stress levels in daily life may prove to be an effective approach to prevention of nicotine addiction.
Acknowledgements
This work was supported by NIH grants R01 DA017288 and R01 DA037277 from the National Institute on Drug Abuse.
References
1. Schepis TS, Rao U. Epidemiology and etiology of adolescent smoking. Curr Opin Pediatr. 2005; 17: 607-612.
2. Finkelstein DM, Kubzansky LD, Goodman E. Social status, stress, and adolescent smoking. J Adolesc Health. 2006; 39: 678-685.
3. West R, Lennox S. Function of cigarette smoking in relation to examinations. Psychopharmacology (Berl). 1992; 108: 456-459.
4. Steptoe A, Wardle J, Pollard TM, et al. Stress, social support and health-related behavior: a study of smoking, alcohol consumption and physical exercise. J Psychosom Res. 1996; 41: 171-180.
5. Orlando M, Ellickson PL, Jinnett K. The temporal relationship between emotional distress and cigarette smoking during adolescence and young adulthood. J Consult Clin Psychol. 2001; 69: 959-970.
6. Siqueira L, Diab M, Bodian C, et al. Adolescents becoming smokers: the roles of stress and coping methods. J Adolesc Health. 2000; 27: 399-408.
7. Niaura R, Shadel WG, Britt DM, et al. Response to social stress, urge to smoke, and smoking cessation. Addictive behaviors. 2002; 27: 241-250.
8. Croghan IT, Bronars C, Patten CA, et al. Is smoking related to body image satisfaction, stress, and self-esteem in young adults? Am J Health Behav. 2006; 30: 322-333.
9. Payne TJ, Schare ML, Levis DJ, et al. Exposure to smoking- relevant cues: effects on desire to smoke and topographical components of smoking behavior. Addictive behaviors. 1991; 16: 467-479.
10. Perkins KA, Grobe JE. Increased desire to smoke during acute stress. Br J Addict. 1992; 87: 1037-1040.
11. Doherty K, Kinnunen T, Militello FS, et al. Urges to smoke during the first month of abstinence: relationship to relapse and predictors. Psychopharmacology. 1995; 119: 171-178.
12. Todd M. Daily processes in stress and smoking: effects of negative events, nicotine dependence, and gender. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors. 2004; 18: 31-39.
13. Parrott AC. Stress modulation over the day in cigarette smokers. Addiction. 1995; 90: 233-244.
14. Koval JJ, Pederson LL, Chan SS. Psychosocial variables in a cohort of students in grades 8 and 11: a comparison of current and never smokers. Prev Med. 2004; 39: 1017-1025.
15. Juliano LM, Brandon TH. Effects of nicotine dose, instructional set, and outcome expectancies on the subjective effects of smoking in the presence of a stressor. Journal of abnormal psychology. 2002; 111: 88-97.
16. Baker TB, Brandon TH, Chassin L. Motivational influences on cigarette smoking. Annu Rev Psychol. 2004; 55: 463-491.
17. Gilbert DG, Robinson JH, Chamberlin CL, et al. Effects of smoking/nicotine on anxiety, heart rate, and lateralization of EEG during a stressful movie. Psychophysiology. 1989; 26: 311-320.
18. Jarvik ME, Caskey NH, Rose JE, et al. Anxiolytic effects of smoking associated with four stressors. Addictive behaviors. 1989; 14: 379-386.
19. File SE, Kenny PJ, Ouagazzal AM. Bimodal modulation by nicotine of anxiety in the social interaction test: role of the dorsal hippocampus. Behav Neurosci. 1998; 112: 1423-1429.
20. George TP, Verrico CD, Xu L, et al Effects of repeated nicotine administration and footshock stress on rat mesoprefrontal dopamine systems: Evidence for opioid mechanisms. Neuropsychopharmacology. 2000; 23: 79-88.
21. Szyndler J, Sienkiewicz-Jarosz H, Maciejak P, et al. The anxiolytic-like effect of nicotine undergoes rapid tolerance in a model of contextual fear conditioning in rats. Pharmacology, biochemistry, and behavior. 2001; 69: 511-518.
22. George TP, Verrico CD, Roth RH. Effects of repeated nicotine pre-treatment on mesoprefrontal dopaminergic and behavioral responses to acute footshock stress. Brain research. 1998; 801: 36-49.
23. Minowa K, Pawlak R, Takada Y, et al. Nicotine attenuates stress-induced changes in plasma amino acid concentrations and locomotor activity in rats. Brain Res Bull. 2000; 51: 83- 88.
24. Pawlak R, Takada Y, Takahashi H, et al. Differential effects of nicotine against stress-induced changes in dopaminergic system in rat striatum and hippocampus. European journal of pharmacology. 2000; 387: 171-177.
25. Buczek Y, Le AD, Stewart J, et al. Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology. 1999; 144: 183-188.
26. Zislis G, Desai TV, Prado M, et al. Effects of the CRF receptor antagonist D-Phe CRF(12-41) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology. 2007; 53: 958-966.
27. Bilkei-Gorzo A, Racz I, Michel K, et al. A common genetic predisposition to stress sensitivity and stress-induced nicotine craving. Biological psychiatry. 2008; 63: 164-171.
28. Bruijnzeel AW, Prado M , Isaac S. Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal- induced deficit in brain reward function and stress-induced relapse. Biological psychiatry. 2009; 66: 110-117.
29. Plaza-Zabala A, Martin-Garcia E, de Lecea L, et al. Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010; 30: 2300-2310.
30. Aghajanian GK, VanderMaelen CP. alpha 2-adrenoceptor- mediated hyperpolarization of locus coeruleus neurons: intracellular studies in vivo. Science. 1982; 215: 1394-1396.
31. Uhde TW, Boulenger JP, Post RM, et al. Fear and anxiety: relationship to noradrenergic function. Psychopathology. 1984; 17: 8-23.
32. Chopin P, Pellow S, File SE. The effects of yohimbine on exploratory and locomotor behaviour are attributable to its effects at noradrenaline and not at benzodiazepine receptors. Neuropharmacology. 1986; 25: 53-57.
33. Abercrombie ED, Keller RW Jr, Zigmond MJ. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience. 1988; 27: 897-904.
34. Holmberg G, Gershon S. Autonomic and psychic effects of yohimbine hydrochloride. Psychopharmacologia. 1961; 2: 93-106.
35. Lang WJ, Gershon S. Effects of psychoactive drugs on yohimbine induced responses in conscious dogs. A proposed screening procedure for anti-anxiety agents. Arch Int Pharmacodyn Ther. 1963; 142: 457-472.
36. Davis M, Redmond DE Jr, Baraban JM. Noradrenergic agonists and antagonists: effects on conditioned fear as measured by the potentiated startle paradigm. Psychopharmacology. 1979; 65: 111-118.
37. Charney DS, Heninger GR, Redmond DE Jr. Yohimbine induced anxiety and increased noradrenergic function in humans: effects of diazepam and clonidine. Life Sci. 1983; 33: 19-29.
38. Bremner JD, Krystal JH, Southwick SM, et al. Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse. 1996; 23: 39-51.
39. Bremner JD, Krystal JH, Southwick SM, et al. Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse. 1996; 23: 39-51.
40. Zarrindast MR, Homayoun H, Babaie A, et al. Involvement of adrenergic and cholinergic systems in nicotine-induced anxiogenesis in mice. Eur J Pharmacol. 2000; 407: 145-158.
41. Schroeder BE, Schiltz CA, Kelley AE. Neural activation profile elicited by cues associated with the anxiogenic drug yohimbine differs from that observed for reward-paired cues. Neuropsychopharmacology. 2003; 28: 14-21.
42. Lee B, Tiefenbacher S, Platt DM, et al. Pharmacological blockade of alpha2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004; 29: 686-693.
43. Shepard JD, Bossert JM, Liu SY, et al. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biological psychiatry. 2004; 55: 1082-1089.
44. Le AD, Harding S, Juzytsch W, et al. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology. 2005; 179: 366-373.
45. Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behavioural brain research. 2006; 174: 1-8.
46. Funk D, Li Z, Le AD. Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: Relationship to the reinstatement of alcohol seeking. Neuroscience. 2006; 138: 235-243.
47. Ghitza UE, Gray SM, Epstein DH, et al. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF1 receptors. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2006; 31: 2188-2196.
48. Nair SG, Gray SM, Ghitza UE. Role of food type in yohimbine- and pellet-priming-induced reinstatement of food seeking. Physiology & behavior. 2006; 88: 559-566.
49. Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000; 33: 13-33.
50. Le A, Shaham Y. Neurobiology of relapse to alcohol in rats. Pharmacology & therapeutics. 2002; 94: 137-156.
51. Lu L, Shepard JD, Hall FS, et al. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev. 2003; 27: 457-491.
52. Bossert JM, Ghitza UE, Lu L, et al. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. European journal of pharmacology. 2005; 526: 36-50.
53. Piazza PV, Deminiere JM, Le Moal M, et al. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989; 245: 1511-1513.
54. Pierre PJ, Vezina P. Predisposition to self-administer amphetamine: the contribution of response to novelty and prior exposure to the drug. Psychopharmacology (Berl). 1997; 129: 277-284.
55. Suto N, Austin JD, Vezina P. Locomotor response to novelty predicts a rat's propensity to self-administer nicotine. Psychopharmacology. 2001; 158: 175-180.
56. Abreu-Villaca Y, Queiroz-Gomes Fdo E, Dal Monte AP, et al. Individual differences in novelty-seeking behavior but not in anxiety response to a new environment can predict nicotine consumption in adolescent C57BL/6 mice. Behavioural brain research. 2006; 167: 175-182.
57. Cain ME, Denehy ED, Bardo MT. Individual Differences in Amphetamine Self-Administration: The Role of the Central Nucleus of the Amygdala. Neuropsychopharmacology. 2008; 33: 1149-1161.
58. Bush DE, Vaccarino FJ, Individual differences in elevated plus-maze exploration predicted progressive-ratio cocaine self-administration break points in Wistar rats. Psychopharmacology. 2007; 194: 211-219.
59. DeSousa NJ, Bush DE, Vaccarino FJ. Self-administration of intravenous amphetamine is predicted by individual differences in sucrose feeding in rats. Psychopharmacology (Berl). 2000; 148: 52-58.
60. Gosnell BA. Sucrose intake predicts rate of acquisition of cocaine self-administration. Psychopharmacology (Berl). 2000; 149: 286-292.
61. LeSage MG, Burroughs D, Dufek M, et al. Reinstatement of nicotine self-administration in rats by presentation of nicotine- paired stimuli, but not nicotine priming. Pharmacology, biochemistry, and behavior. 2004; 79: 507-513.
62. Cohen C, Perrault G, Griebel G, et al. Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716). Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2005; 30:
145-155.
63. Paterson NE, Froestl W, Markou A. Repeated administration of the GABAB receptor agonist CGP44532 decreased nicotine self-administration, and acute administration decreased cue-induced reinstatement of nicotine-seeking in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2005; 30:119-128.
64. Liu X, Caggiula AR, Yee SK, Mecamylamine attenuates cue-induced reinstatement of nicotine-seeking behavior in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007; 32:710-718.
65. Corrigall WA. A rodent model of nicotine self-administration. In Boulton, A., Baker, G., Wu, P.H. (eds) Animal models of drug addiction: Neuromethods. Humana Press. 1992; 315-344.
66. Shoaib M, Schindler CW, Goldberg SR. Nicotine self- administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology. 1997; 129: 35-43.
67. Donny EC, Caggiula AR, Mielke MM, et al. Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology. 1998; 136: 83-90.
68. Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N Engl J Med. 1994; 331: 123-125.
69. Matta SG, Balfour DJ, Benowitz NL, et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology. 2007; 190: 269-319.
70. Levin ED, Slade S, Wells C, et al. D-cycloserine selectively decreases nicotine self-administration in rats with low baseline levels of response. Pharmacology, biochemistry, and behavior. 2011; 98: 210-214.
71. Bilkei-Gorzo A, Racz I, Michel K, et al. A Common Genetic Predisposition to Stress Sensitivity and Stress-Induced Nicotine Craving. Biol Psychiatry. 2007;
72. Erblich J, Boyarsky Y, Spring B, et al. A family history of smoking predicts heightened levels of stress-induced cigarette craving. Addiction. 2003; 98: 657-664.
73. Colamussi L, Bovbjerg DH, Erblich J. Stress- and cue-induced cigarette craving: effects of a family history of smoking. Drug and alcohol dependence. 2007; 88: 251-258.
74. Buchmann AF, Laucht M, Schmid B, et al. Cigarette craving increases after a psychosocial stress test and is related to cortisol stress response but not to dependence scores in daily smokers. J Psychopharmacol. 2010; 24: 247-255.
75. Childs E, de Wit H. Effects of acute psychosocial stress on cigarette craving and smoking. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2010; 12: 449-453.
76. Piazza PV, Maccari S, Deminiere JM, et al. Corticosterone levels determine individual vulnerability to amphetamine self- administration. Proc Natl Acad Sci USA. 1991; 88: 2088-2092.
77. Mantsch JR, Saphier D, Goeders NE. Corticosterone facilitates the acquisition of cocaine self-administration in rats: opposite effects of the type II glucocorticoid receptor agonist dexamethasone. The Journal of pharmacology and experimental therapeutics. 1998; 287: 72-80.
78. Goeders NE. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology. 2002; 27: 13-33.
79. Lee B, Tiefenbacher S, Platt DM, et al. Role of the hypothalamic- pituitary-adrenal axis in reinstatement of cocaine-seeking behavior in squirrel monkeys. Psychopharmacology. 2003; 168: 177-183.
80. Sinha R, Garcia M, Paliwal P, et al. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006; 63: 324-331.
81. Mello NK. Hormones, nicotine, and cocaine: clinical studies. Horm Behav. 2010; 58: 57-71.
82. Sharp BM, Beyer HS. Rapid desensitization of the acute stimulatory effects of nicotine on rat plasma adrenocorticotropin and prolactin. The Journal of pharmacology and experimental therapeutics. 1986; 238: 486-491.
83. Pomerleau OF, Pomerleau CS. Cortisol response to a psychological stressor and/or nicotine. Pharmacology, biochemistry, and behavior. 1990; 36: 211-213.
84. Matta SG, Foster CA, Sharp BM. Nicotine stimulates the expression of cFos protein in the parvocellular paraventricular nucleus and brainstem catecholaminergic regions. Endocrinology. 1993; 132: 2149-2156.
85. Matta SG, Fu Y, Valentine JD, et al. Response of the hypothalamo-pituitary-adrenal axis to nicotine. Psychoneuroendocrinology. 1998; 23: 103-113.
86. Sharp BM, Matta SG. Detection by in vivo microdialysis of nicotine-induced norepinephrine secretion from the hypothalamic paraventricular nucleus of freely moving rats: dose-dependency and desensitization. Endocrinology. 1993; 133: 11-19.
87. Baron JA, Comi RJ, Cryns V, et al. The effect of cigarette smoking on adrenal cortical hormones. The Journal of pharmacology and experimental therapeutics. 1995; 272: 151-155.
88. Frederick SL, Reus VI, Ginsberg D, et al. Cortisol and response to dexamethasone as predictors of withdrawal distress and abstinence success in smokers. Biological psychiatry. 1998; 43: 525-530.
89. Donny EC, Caggiula AR, Rose C, et al. Differential effects of response-contingent and response-independent nicotine in rats. European journal of pharmacology. 2000; 402: 231-240.
90. Chen H, Fu Y, Sharp BM. Chronic nicotine self-administration augments hypothalamic-pituitary-adrenal responses to mild acute stress. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008; 33: 721-730.
91. Reich T, Hinrichs A, Culverhouse R, et al. Genetic studies of alcoholism and substance dependence. American journal of human genetics. 1999; 65: 599-605.
92. Vanyukov MM, Tarter RE. Genetic studies of substance abuse. Drug and alcohol dependence. 2000; 59: 101-123.
93. Uhl GR, Liu QR, Naiman D. Substance abuse vulnerability loci: converging genome scanning data. Trends Genet. 2002; 18: 420-425.
94. Rhee SH, Hewitt JK, Young SE, et al. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Archives of general psychiatry. 2003; 60: 1256-1264.
95. Pomerleau OF, Pomerleau CS. Research on stress and smoking: progress and problems. Br J Addict. 1991; 86: 599- 603.
96. Pauly JR, Grun EU, Collins AC. Tolerance to nicotine following chronic treatment by injections: a potential role for corticosterone. Psychopharmacology. 1992; 108: 33-39.
97. Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997; 278: 52-58.
98. Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001; 158: 343-359.
99. Zinser MC, Baker TB, Sherman JE, et al. Relation between self-reported affect and drug urges and cravings in continuing and withdrawing smokers. Journal of abnormal psychology. 1992; 101: 617-629.
100. McKee SA, Sinha R, Weinberger AH. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. J Psychopharmacol. 2011; 25: 490-502.
101. Piazza PV, LeMoal M. Pathophysiological basis of vulnerability to drug abuse: Role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu. Rev. Pharmacol. Toxicol. 1996; 36: 359-378.
102. Koob GF. Stress, corticotropin-releasing factor, and drug addiction. Annals of the New York Academy of Sciences. 1999; 897: 27-45.
103. Goeders NE. Stress and cocaine addiction. The Journal of pharmacology and experimental therapeutics. 2002; 301:785-789.
104. Sinha R. Chronic stress, drug use, and vulnerability to addiction. Annals of the New York Academy of Sciences. 2008; 1141: 105-130.