The Effectiveness and Tolerability of Globifer Forte (Haem Iron) Tablets on Iron Deficiency Anaemia in Pregnancy
Author'(s): Elmahaishi Wael M, Zwawa Alia A, Elmahaishi Asma M and Elmahaishi MS*
Lamis Clinic and Said Hospital for Obstetrics and Gynecology, Misurata Libya.
*Correspondence:
Elmahaishi MS, Department of Obstetrics and Gynecology, Lamis Clinic, Said Private Hospital, Libya, E-mail: Elmahaishi@elmahaishi.com.
Received: 21 November 2017 Accepted: 24 December 2017
Citation: Elmahaishi Wael M, Zwawa Alia A, Elmahaishi Asma M, et al. The Effectiveness and Tolerability of Globifer Forte (Haem Iron) Tablets on Iron Deficiency Anaemia in Pregnancy. Gynecol Reprod Health. 2017; 1(4): 1-4.
Abstract
Background: Anemia caused by Iron Deficiency, is described as reduced amount of red blood cells (RBCs) or decreased hemoglobin as a result of lower level of iron in the human body. We carried out this study to evaluate the effectiveness of Globifert fort (oral iron intake) in iron deficiency anemia (IDA) in pregnant patients.
Methods: In this prospective study 100 pregnant women were involved. All of them were given Globifert fort tables during the study period of 12 weeks.
Results: Hb level have reached the target level within a short period of time with very minor side effects or discomfort.
Conclusions: Globifert Fort has been found very suitable, effective and convenient in treatment of IDA during antenatal care.
Keywords
Introduction
Iron deficiency anemia (IDA), is introduced as an insufficient iron concentrations in the human body that lead to reduction in the level of red blood cells (RBCs) or Hemoglobin (HB) in the blood [1].
In developing countries, anemia is estimated to affect approximately more than half the women population. Anemia caused by Iron deficiency is the most responsible during pregnancy. Based on WHO reports, IDA is estimated to affect nearly 18% in developed countries and about 56% in Developing countries. Worldwide, the percentage of IDA is estimated to be about 55.9% [2].
World Health Organization describes anemia as HB level under 11g%2 in pregnant women and below 7g%2 is considered a sever form. Serum ferritin less than 15 microgram/l is reflected as IDA [3]
The commonest nutritional deficiency affecting pregnant women is IDA [4]. This Deficiency may result from several factors including low iron content in the diets together with menstruations and an unplanned pregnancy. In pregnancy, there is a large need for iron to meet the demand of red blood cell mass expansion in the mother, fetal and placental blood and blood loss during delivery time. ID is exaggerated due to the ability of fetus to extract its demands. This is aggravated by poor absorption of iron as a result of the adverse effects of pregnancy on the gastrointestinal system such as nausea and vomiting, motility disorder with reflux esophagitis and indigestion [5].
Globally, anemia is a major public health issue that ID is the main contribution to it. In developing countries, IDA has a great impact on maternal morbidity and mortality [4].
Over the previous years, various mode of iron introduction has been used such as oral, injections and intravenous for managing IDA during pregnancy. However, these methods are involved with noteworthy side effects and the impossibility to increase HB levels to the required level in a short period of time, in particular third trimester. Recently, Globifert forte is a relatively a new drug that is used orally for the correction of anemia and IDA. Oral intake of Globifert forte has its limitations. Blood transfusion is the last resort and can be used only in severe cases of symptomatic anemia [6,7]. Although blood transfusion can reliably treat anemia, it has its disadvantages and limitations. Considering the advantages and limitations of the available options, Globifert forte tablets is safe, convenient and more effective other types in treating IDA. It has been able to increase HB level to acceptable level when applied during pregnancy.
The aim of this study was to assess the efficacy of Globifert forte in pregnant women with IDA.
Methods
This is a prospective, single centre, open and non-comparator study in Lamis Clinic and Said Hospital, Misurata, Libya. We included a total of 100 pregnant ladies with IDA for a time period of 12 weeks. The treatment administered was Globifer Forte, intake twice daily each day until completion of the trial. Each tablet contains: haemoglobin powder 600 mg, 18 mg Fe2+ as heme iron.
The administration of GlobiFer Forte leads to a clinical relevant increase of 1g/dL of the haemoglobin level of the patients with iron deficiency anaemia. The endpoints are the rates of patients achieving the objective after 4, 8 and 12 weeks.
Exclusion criteria
- History of acquired iron overload, known hemochromatosis.
- Known allergy to oral iron preperations.
- Diseases, which an iron supplementation is not allowed or contraidicated.
- Patients with abnormal MCV (>100 fL) and high MCHC (> 36 g/dL).
- Patients on current oral or intravenous iron supplementation
- History of erythropoietin therapy in previous 30 days or scheduled for erythropoietin therapy or blood transfusion during duration of the study.
- Patients who have had iron supplementation within the last 3 months.
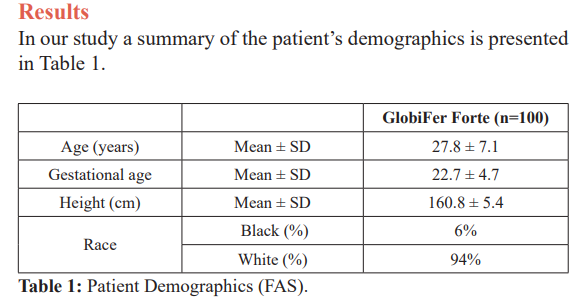
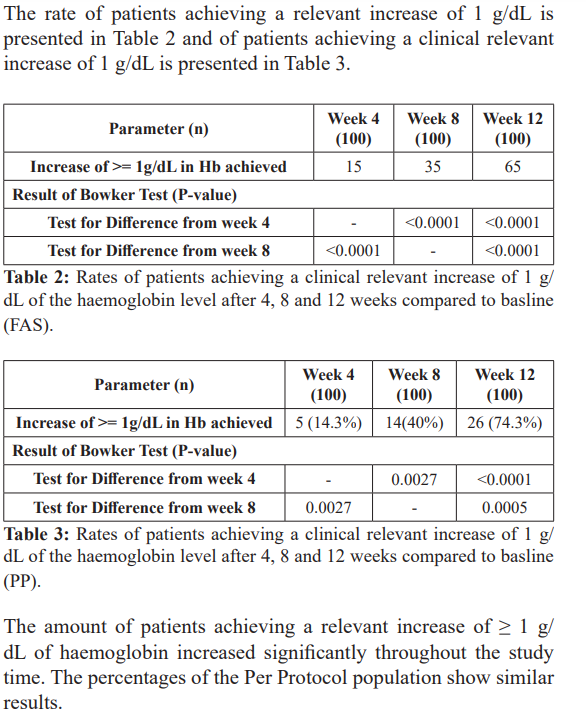
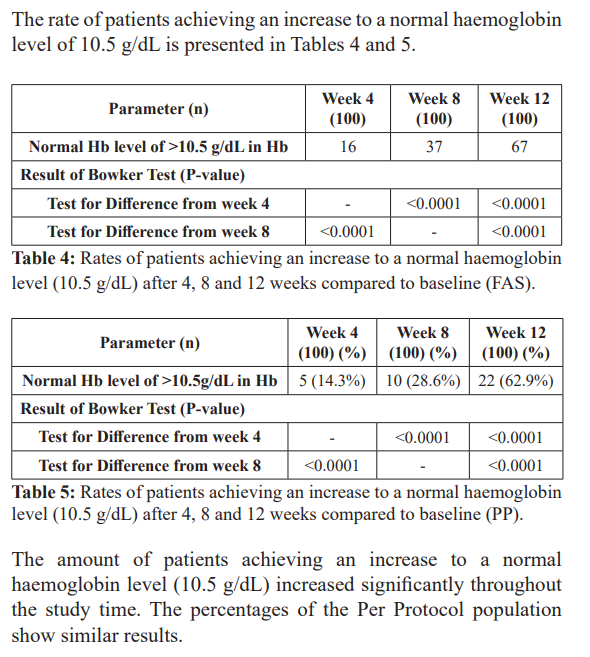
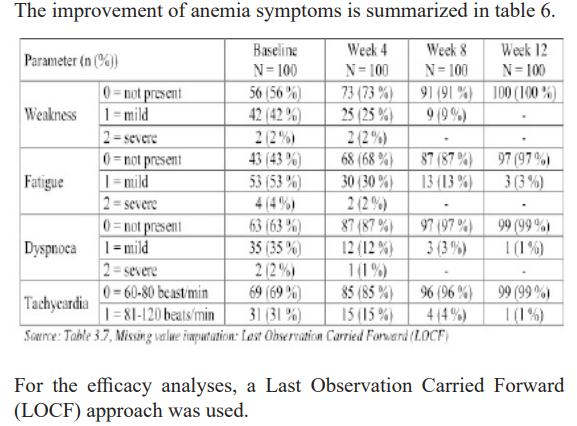
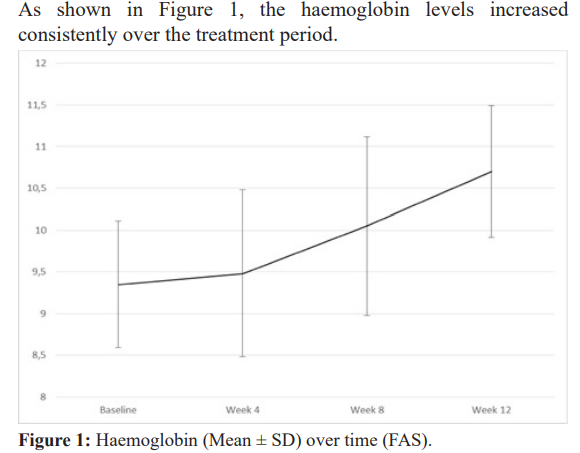
The trial was able to show that the haemoglobin levels increased continuously and an increasing amount of responders (both for increase in haemoglobin level of 1 g/dL and reaching a haemoglobin level of 10.5 g/dL). The anaemia symptoms improved over the 12 weeks period, with nearly no symptoms left at the last observation point.
Discussion
The fetus and placenta demand nearly 500 mg of iron and a parallel quantity is required for red cell increment. An average postpartum blood loss and lactation for six months each accounts for about 180 mg. From total of 1360 mg, 350 mg may be subtracted (saved as a result of amenorrhea) to give an actual extra demand for about 1000 mg. This is unlikely to be provided by dietary iron but may be mobilized from full iron stores (about 1000 mg). It is the state of stores that largely determine whether or not a pregnant woman becomes anemic. The smaller her stores, the earlier the anemia occurs [11].
Treatment of IDA has included oral iron, intramuscular, iron dextran, ISC, Recombinant erythropoietin and blood transfusion. However, most of these have their disadvantages. Although antenatal oral iron supplements has been in practice for long time, pregnant ladies who respond well to the therapy demands a long time (months) to correct the Hb level and has lack of compliance which is mainly due to the effect on gastro intestinal system. Compared with parenteral application, it requires weeks to replenish the dropped iron stores. However, the correction speed of Hb status is parallel to that with oral intake. Also, due to the high cost of parentral iron treatment and the risk of complications such as anaphylaxis, pain and staining, its applications in managing Hb status has been limited to selected pregnancies [8-10].
In the current study, side effects of oral iron supplements had not been seen during the study time such as gastrointestinal effect, thus showing the safety of the drug in the pregnant women and its acceptance from the patients. One tablet twice a day was the maximum therapeutic effect that could raise the Hb level to the desired point and one tablet a day is the Prophylactic dose aganist IDA during pregnancy. Any increase in the number of tablets a day more than the recommended dose will not prove the Hb level any faster or higher. Thus our study showed that iron tablets could be used in the pregnant ladies with iron deficiency anemia. These results made this oral supplement superior to other types of oral iron therapy and more desirable than intravenous iron injections with respect to faster increase in Hb and faster replenishment of body iron stores with little side effects [9]. Furthermore, it decreases the demand for blood transfusions [12].
Conclusion
Out of 100 patients, the maximum number of patients that acquired the target Hb level was after 12 weeks therapy. In all of them Hb level correction progress was steady improved through the therapy period.
In this study we found side effects of iron therapy intake and anemia symptoms had been nearly disappeared in almost every pregnant patient on therapy. Moreover, in all of pregnant patients, the Hb level had improved by an average >1g/dL through the therapy.
This study revealed significant improvement of anemia as Hb level and its symptoms in pregnant women given calculated dose of Globifert forte tablets. It was safe, tolerable and well accepted. This kind of therapy could be valuable and beneficial in management of cases with commonly detected IDA in pregnancy in our country.
References
- Provan D. Mechanism and management of iron deficiency Br J Haematol. 1999; 105: 19-26.
- Dutta DC. Anaemia in pregnancy. In Textbook of Obstetrics including Perinatology & Contraception. 6th ed. Calcutta, India New Central Book Agency P 2004; 262-267.
- Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information Geneva, World Health Organization. 2011.
- UNICEF and Micronutrient Initiative. Vitamin and mineral deficiency a global progress 2004.
- Panel on Micronutrients, Food and Nutrition Board, Institute of Medicine-National Academy of Dietary reference intakes recommended intakes for individuals, vitamins. 2001.
- Milman N, Bergholt T, Byg K, et al. Iron status and iron balance during pregnancy, a critical reappraisal of iron Acta obstet gynaecol Scand. 1999; 78: 749- 757.
- Mahomed Iron and folate supplementation in pregnancy. In the Cochrane library. Oxford update. 2002.
- Bhandal N, Russel R. Intravenous versus oral iron therapy in postpartum BJOC. 2006; 113: 1248-1252.
- Bashiri A, Burstein E, Sheiner E, et Anaemia during Pregnancy and treatment with intravenous iron review of the literature. Eur J Obstet Gynecol Reprod Biol. 2003; 110: 2-7.
- Krafft A, Perewusnyk G, Hanseler E, et al. Effect of postpartum iron supplementation on red cell and iron parameters in nonanaemic iron deficient women: a randomized placebo- controlled BJOG. 2005; 112: 445-450.
- Centre for disease Criteria for anemia in children and childbearing aged women. MMWR. 1989; 38: 400-404.
- Hallak M, Sharon A, Duikman R, et al. Supplementing iron intravenously in A way to avoid blood transfusions. J Reprod Med. 1997; 42: 99-103.