The Renaissance of Hormone-Free Barrier Contraception and Development of FemCap™: Hormone-Free Contraception
Author(s): Shihata Alfred1, Steven A. Brody MD2 and Julia Barrett-Mitchell3
1Scripps Institution of Medicine and Science San Diego, CA, USA Phone: (858) 922-7673.
2Director of Life Span Medical Institute San Diego, CA, USA.
3FemCap Inc., Clinical coordinator, 14058 Mira Montana Drive Del Mar CA 92014.
*Correspondence:
Shihata Alfred, Scripps Institution of Medicine and Science San Diego, CA, USA Phone: (858) 922-7673.
Received: 13 August 2020 Accepted: 01 September 2020
Citation: Alfred S, Brody SA, Julia Barrett-Mitchell J. The Renaissance of Hormone-Free Barrier Contraception and Development of FemCap™: Hormone-Free Contraception. Gynecol Reprod Health. 2020; 4(4): 1-6.
Abstract
The objectives of this review to trace the history of female barrier contraceptive devices, and highlight the recent advances, in the field to be able to plan safer and more effective, devices in the future. We conducted a literature search of the history of female barrier contraceptive methods. Cervical barriers such as Prentif cap, Vimule and Dumas Cap could not survive the market despite FDA approval of Prentif Cap. The diaphragm and cervical cap were the only contraceptives available for women. They became outdated once hormonal methods became available. The pill is easy to use; however, hormones have many undesirable side effects. There is a recent surge in demand by women who want to avoid the adverse effects of hormones, and switch back to the safest and most natural methods of contraception. The safety and the potential efficacy of barrier methods inspired the author to invent the FemCap. We conducted a critical review of the currently available barrier methods to overcome their drawbacks and improve upon their design. We changed the material used to produce these devices from Latex to medical grade Silicone, which is hypoallergenic and durable. To produce an ergonomically fitting device, the anatomy of the cervix and vagina were traced. This is to conform to cervical anatomy and to fit the cervix like a glove and adapt to the physiological changes in the cervix and vagina during the menstrual cycle, and the changes that occur during vaginal delivery. Research continued after the FDA approval of the FemCap to find other uses of the FemCap such as control of stress incontinence and using the FemCap to enhance the fertility awareness methods. Barrier contraceptive devices are limited, and more options are needed. Female barrier devices play an important role for women who have contraindications or aversion to hormonal methods.
Keywords
Introduction
A literature search in the cervical barrier in general and cervical caps in particular showed women were concerned about limiting their family size and seeking some form of contraception since the beginning of time. It was recorded on Egyptian Papyrus (1550 BC) that ancient Egyptian women used animal dung, as vaginal pessaries to prevent pregnancy. In the medieval times European women used half a lemon to cover their cervix to prevent pregnancy.
This modality is the closest to modern cervical cap while the rind of the lemon act as a mechanical barrier and the lemon juice act as spermicide. The Prentif-cavity rim cervical cap was invented in 1838 (Figure 1).
FemCap advantages over the Diaphragm
FemCap is designed anatomically to conform to cervix and vagina. It is made of a sigle-piece that heat and cools uniformly allowing for autoclave sterlization V.S the diaphragm that is designed like cup with disregard to the anatomy. It is that made of rubber and stainless steel spring in the rim to keep in the rounded shape.
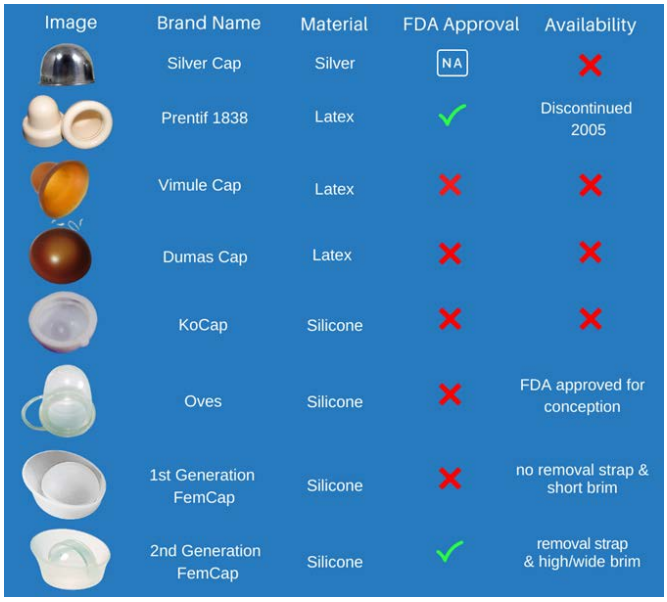
Figure 1: Development of Cervical Caps
The brim of the FemCap flare outwards against the inwards contraction of the vagina causing the brim of the FemCap to adher and conform to vaginal walls creating a tight seal, without causing any undue preasure over the the vagina or urethra. This in stark contrast to the rigid rim of the diaphram that kink the urethra which causes partial obstruction and increased incidence of urinary tract infection.
The FemCap is designed with unique groove facing the vaginal opening that act as trap for sperms and as a storage for spermicide or future microbicide. This versus the diaphragm, where the spermicide is applied facing the cervix which disrupt and damage the delicate single layer of endocervical canal.
The FemCap is designed with a removal strap across the dome eliminating the potential fingernai abrasion during removal. This is versus the diaphragm that fingernail abrasion can occur during removal.

Figure 2: Diaphragm.
FemCap Size selection depends on the obestetical history without the need of laborous fitting needed for the diaphragm.
The FemCap provide protection for 48 hours Versus the diaphragm that is not recommended for over 24 hours (Figures 2, 3).

Figure 3: FemCap.
FemCap™ Discription and Its Evolution
The obsolete first-generation FemCap was designed to conform to the anatomy of the cervix and to adapt to the physiology of the vagina [1-3].
In the second-generation design [4,5,6] the DOME covers the cervix completely (Figure 5, 9). The RIM (Figure 5, 15) fits snugly into the vaginal fornices. The RIM (Figure 5, 15) encircles the opening of the FemCap that fits over the cervix. A LIP (Figure 5) was created inside the rim to gently grip the cervix. The BRIM (Figure 5, 15) was designed to flare outward so it would push against the physiological inward contraction of the vagina. This pressure/ counter-pressure causes the BRIM (Figure 5, 15) to adhere and conform to the vaginal walls creating a tight seal. In keeping with vaginal anatomy, the BRIM was designed to be longer posteriorly (Figure 5). The FemCap is designed with a GROOVE (Figure 5). This groove is intended to store any spermicide or microbicides that may be developed in the future to protect against sexully transmitted infections.
Size selection
Because all women are unique in anatomy it only made sense one size could not fit all. We started with 3 sizes, the smallest 22mm is designed for womem who have never been pregnant, the medium 26mm for women who have been pregenant but did not deliver vaginally such as C-section or miscarriage and the large 30mm is for women who have delivered vaginally.
The utilization of the 3 sizes eliminated the need of time consuming measurment and custom fitting. Instead size selection is determined by the womans obestitrical history.
The most striking observation of this study is that the diamter of the cervix has very little relation to height and weight. The ONLY factors having a major impact on the cervical diameter and the elastisity of the vagina are pregnancy and delivery.
An initial concern with the first- generation FemCap was; would it be too difficult for women to correctly place the FemCap over a tilted cervix? To remedy this we designed an applicator to place the FemCap correctly. Actual data showed that women in fact didn’t have trouble placing the FemCap over the cervix even if tilted however, they did have difficulty in removeing it. Since the insertion wasn’t an issue, the applicator became obsolete.
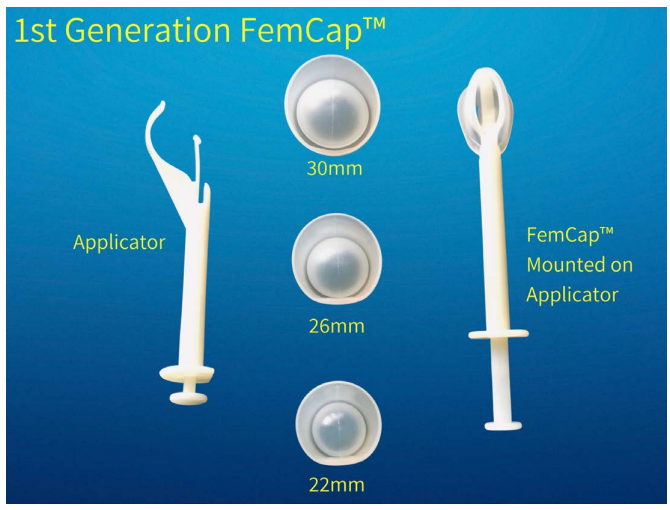
Figure 4: First Generation FemCap with Applicator.
To ease the difficulty when removing the FemCap we designed a new version known as the second generation. The second generation FemCap is currently the only cervical cap available in the entire world with FDA & CE approval (Figure 2-11). A strap was added over the dome of the FemCap (Figure 11 ) to alleviate the difficult removal.
The first generation FemCap also had a lowered rate of effectiveness for women who had given birth vaginally. This reduced efficacy was due to a decline in vaginal tone, which increased dislodgment. To compensate for the decreased vaginal tone in multiparous women we increased the dimension of the brim to maximize the surface contact between the vaginal walls and the FemCap. This helped to improve the stability, prevent dislodgment and enhance effectiveness.
The FemCap is designed with a unique groove facing the vaginal opening (Figure 5). This groove is intended to trap sperm while keeping the spermicide from touching the cervix to minimize any possible irritation. The bulk of spermicide/ microbicide is intended to meet the sperm, bacteria, and/or viruses as soon as they are deposited in the vagina to prevent sexually transmited infections. This is unlike the diaphragm and Prentif cervical cap, in which the spermicide is applied directly against the cervix.
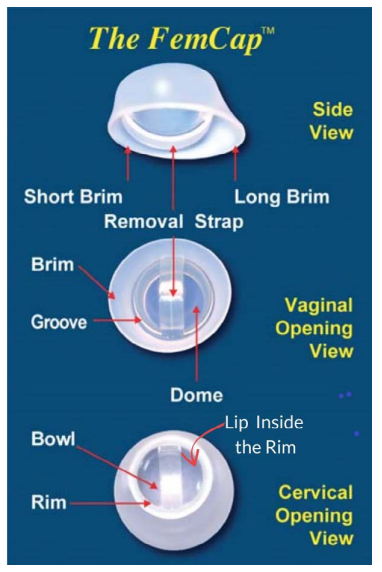
Figure 5: FemCap Views Diagram.

Figure 6: FemCap.
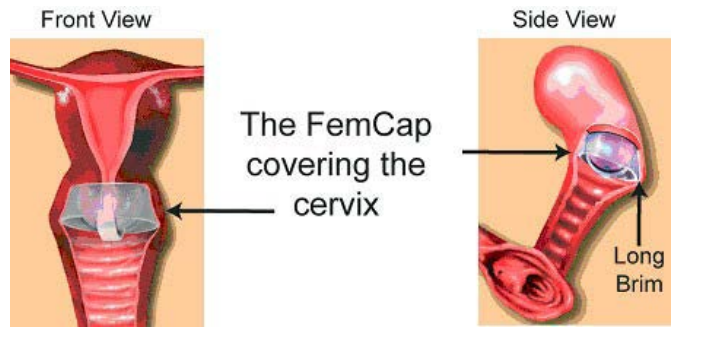
Figure 7: FemCap Covering Cervix.
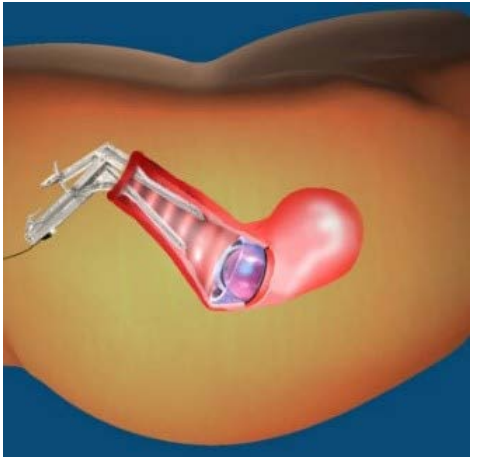
Figure 8: Speculum View.
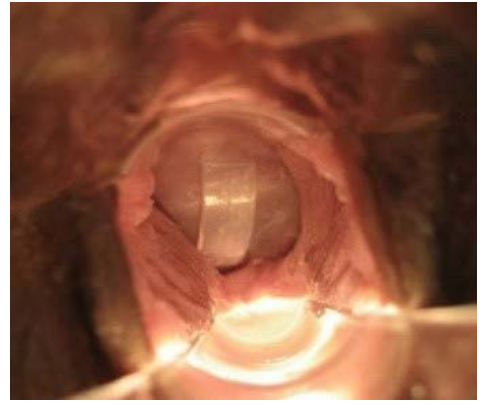
Figure 9: FemCap Over the Cervix.
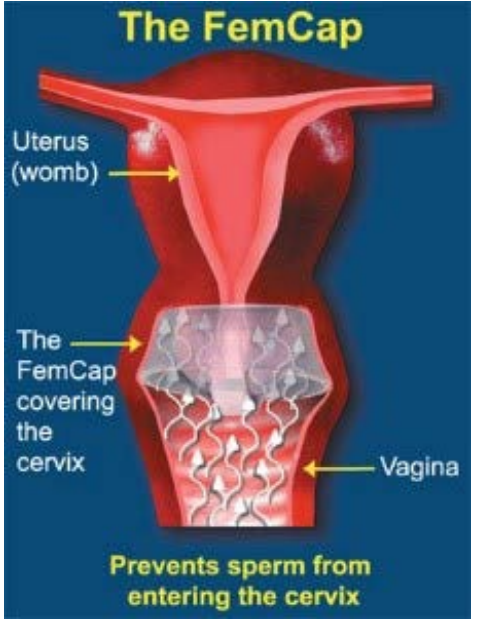
Figure 10: How the FemCap works.
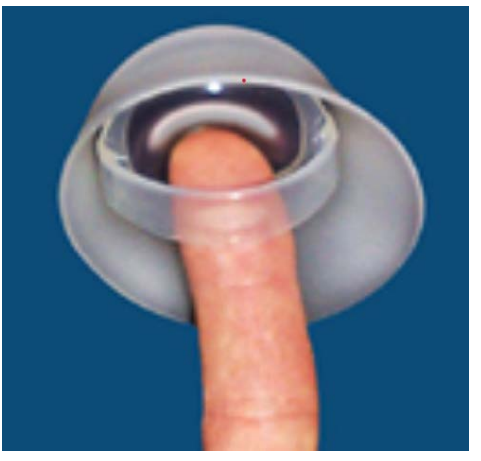
Figure 11: Using the Removal Strap.
Second Generation FemCap Safety, Effectiveness, and Acceptability
During the extensive clinical trials in 10 universities across the United States and two decades of use in the market, the FemCap did not have any reported significant side effects. The second generation FemCap was found to be 92.4% effective in preventing pregnancy [4,5,6]. In terms of the effectiveness of the second generation FemCap, one pregnancy occurred among 85 women who completed 8 weeks of study. Because of the small number of participants and the relatively short duration, the confidence interval is wide. Based on this study, the typical failure rate (Pearl index) of the second generation FemCap is approximately 7.6 per 100 women per year. The effectiveness rate of the FemCap could be much higher for women who are highly motivated and who use the FemCap consistently before each act of intercourse and before sexual arousal [7]. The FemCap is highly acceptable. In these clinical trials 75% of women preferred the FemCap over the diaphragm [3].
The Fertility Awareness Method is the safest and the most cost- effective of all contraceptives, yet it is the least prescribed by doctors and the least used by women [8-15] We attribute this to the fact that women miss the most important sign of ovulation during their fertile window (Figure 12), which is the fertile cervical mucus (Figure 13). The FemCap allows women to collect a high-quality sample of their fertile cervical mucus directly from the source. The FemCap also prevents the fertile cervical mucous from mixing with other vaginal secretions [8]. We previously conducted a pilot study using the FemCap which allowed women to see the distinction of the mucus. It resembles clear raw egg-white and stretches about 2.5 - 3 inches before it breaks. This is the most important step in identifying ovulation and the fertile window with astonishing precision. This methodology shortened the fertile window to 3 days for conception and 8 days for contraception. This simple non-invasive and low-cost method can maximize the chance of conception or contraception in healthy women having regular periods. It should be noted that the efficacy of this method depends intensively on user motivation, compliance, and accurate, consistent recording.
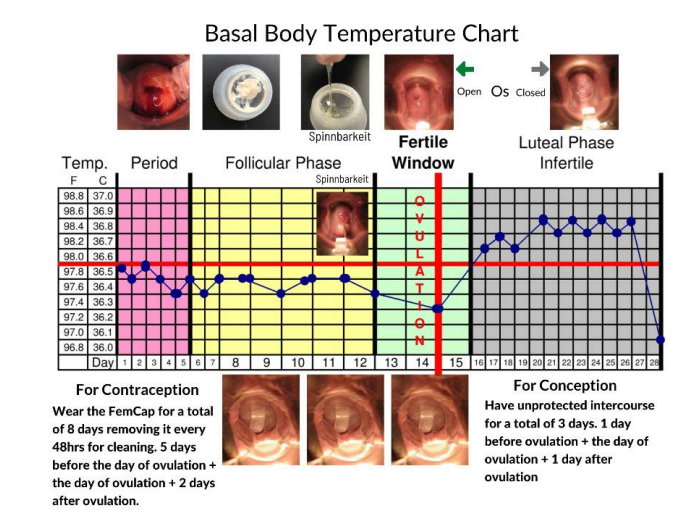
Figure 12: FemCap and Fertility Awareness.

Figure 13: Cervical Mucus Collected by FemCap.
The use of the FemCap to collect the cervical mucous can pinpoint the day ovulation and thereby enhance Fertility Awareness methods.
New use for the FemCap is a welcomed addition
A woman who was using the FemCap for contraception reported to me that she was also suffering from Stress Urinary Incontinence (SUI). She reported to me that the days she used the FemCap she did not have any episode of stress incontinence. This led me to investigate the use of the FemCap as a SUI pessary (Figure 15). Stress Urinary Incontinence (SUI) is very prevalent among women of all ages, particularly menopausal women. SUI is under- reported by women as well as under-diagnosed and under-treated by doctors. The first line of SUI treatment is pelvic floor muscle (Kegel) exercises and vaginal pessaries. The ring pessary is most widely used however, more pessaries of different shapes and sizes (Figures 14, 16) have been introduced into the market with the hope of achieving better results.

Figure 14: Pessaries for SUI.
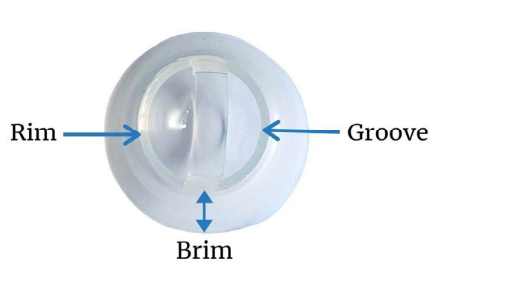
Figure 15: Rim & Brim of FemCap.

Figure 16: Ring Pessary.
Currently available pessaries have significant limitations such as displacement, erosion or even ulceration and urethral obstruction [16-19]. The FemCap shows marked similarity to the ring pessary. The Rim of the FemCap is similar in shape and function to the ring pessary that supports the bladder neck. The outward flaring brim restores the anatomy of the urethra and the vagina. The bowl of the FemCap supports the cervix and prevents it from descending, which provides further support. The FemCap’s rim is like the ring pessary in shape and function to support the bladder neck. The brim flares outward to support and restore the urethra's anatomy and the vagina. Lastly, the bowl of the FemCap supports the cervix to prevent it from descending. We conducted a pilot clinical trial to check the feasibility of the FemCap in controlling stress urinary incontinence. 16 women out of 19 were completely dry [20]. It would be ideal and cost-effective for women to acquire one multipurpose device that can be used for contraception, to control stress incontinence, and enhance fertility awareness methods.
Summary
The currently available intravaginal barrier contraceptive devices are extremely limited, and more options are urgently needed. Female barrier devices play an especially important role for women who have contraindications or aversion to hormonal methods or IUDs. The FemCap is designed to fulfill the current and future needs for barrier methods of birth control. It was designed keeping in mind ease of use, minimal one-on-one training from the health care professional, and possible future use with microbicidal spermicides-a major departure from the currently available diaphragm and cervical cap.
Acknowledgements
This paper was prepared at the request of James D. Fett Editor and Naina K Editorial Assistant, Gynecology & Reproductive Health, we are indebted to the woman who brought this new usage information to our attention, to the women who consented to participate in this research study and to Dr. Steven Brody who co-authored and edited this manuscript. No funding was requested or received from any institution. The authors are responsible for the content of this article.
References
- Alfred A Shihata, James Trussell. New Female Intravaginal Barrier Contraceptive Device Preliminary Clinical Trial. Contraception. 1991; 44: 11-19.
- Mauck C, Baker J, Barr S, et Phase I study of FemCap used with and without spermicide: Postcoital testing. Contraception. 1997; 56: 111-115.
- Mauck C, Callahan M, Weiner D, et al. A comparative study of the safety and efficacy of FemCap,'I: Ma new vaginal barrier contraceptive, and the Ortho All-Flex® diaphragm. Contraception. 1999; 60: 71-80.
- Carcio H, Clarke Secor M, Koeniger-Donohue R. Advanced Health Assessment of Women: Clinical Skills and Procedures Chapter 15 The FemCap. Springer Publishing Company. 2010; 271-278.
- Koeniger-Donohue R. The FemCap a Non-Hormonal Women’s Health Care. NPWH. 2006; 5: 79-91.
- Shoupe D, Kjos S. The Handbook of Contraception, Barrier Contraceptives Chapter 10 Humana Press 2006 Pages 147-17 Shihata A, The FemCap™, a new contraceptive choice. Eur J Contracept and Reprod Health Care. 1998; 3: 160-166.
- Shihata The FemCap™, a new contraceptive choice. Eur J Contracept and Reprod Health Care. 1998; 3: 160-166.
- Shihata Alfred, Brody Steven. Novel approach for women to identify the precise day of Ovulation to conceive or International Journal of Medical Science and Health Research. 2020; 4: 3.
- Pyper Fertility Awareness & Natural Family Planning. Eur J Contracept Reprod Health Care. 1997; 2: 131-146.
- Billings Cervical mucus: The biological marker of fertility and infertility. International J Fertil. 1987; 26: 181.
- The Infertile Period-Principles and Practice. London, Darton, Longman and Todd. 1963.
- Clubb E, Knight J. Fertility–Fertility Awareness and Natural Family Planning. 3rd ed. David & Charles. 1996.
- Arevalo M, Sinai I, Jennings A fixed formula to define the fertile window of the menstrual cycle as the basis of a simple method of NFP. Contraception. 1999; 60: 357-360.
- Odeblad Cervical mucus and their functions. J Irish Coll Phys Surg. 1997; 26: 1.
- Fordney-Settlage D. A review of cervical mucus and sperm interactions in humans. Int J Fertil. 1981; 26: 161-169.
- Shihata A. New FDA approved woman-controlled, latex- free barrier contraceptive device “FemCap™”. Fertil Steril. International Congress Series. 2004; 103: 303-330.
- Viera AJ, Larkins-Pettigrew M. Practical use of the pessary Am Fam Physician. 2000; 61: 2719-2729.
- Al-Shaikh G, Syed S, Osman S, et al. Pessary use in stress urinary incontinence: a review of advantages, complications, patient satisfaction, and quality of life. Int J Womens Health. 2018; 10: 195-201.
- Jones K, Harmanli Pessary Use in Pelvic Organ Prolapse and Urinary Incontinence. Reviews in Obstetrics & Gynecology. 2010; 3: 1-10.
- Viera A, Pettigrew Practical Use of the Pessary. American Family Physican. 2000; 61: 2719-2726.
- Shihata Alfred, Brody Multipurpose, Reusable, Female Contraceptive Device That Enhances the Effectiveness of Fertility Awareness Methods and Controls Stress Incontinence. Medical Research Archives. 2020; 8.