Utility of Preimplantation Genetic Screening in Women of Advanced Maternal Age with Poor Embryo Survival: A Case Report
Author'(s): Angela Stephens, M.D1*, Jessica Kanter, M.D2and Larisa P. Gavrilova-Jordan, M.D3
1Augusta University, Department of Obstetrics and Gynecology,Division of Reproductive Endocrinology, Infertility, and Genetics: Resident Physician, Augusta, GA, USA.
2Resident Physician, Augusta University, Department of Obstetrics and Gynecology, Division of Reproductive Endocrinology, Infertility, and Genetics, GA, USA.
3Associate Professor and Director of In Vitro Fertilization and Fertility Preservation Program, Augusta University, Department of Obstetrics and Gynecology, Division of Reproductive Endocrinology, Infertility, and Genetics, GS, USA.
*Correspondence:
Angela Stephens, Resident Physician, Augusta University, Department of Obstetrics and Gynecology, Division of Reproductive Endocrinology, Infertility, and Genetics, Augusta, GA 30912, USA, Tel: 706-847-8343; E-mail: anstephens@ augusta.edu.
Received: 09 February 2018; Accepted: 10 March 2018
Citation: Angela Stephens, Jessica Kanter, Larisa P. Gavrilova-Jordan. Utility of Preimplantation Genetic Screening in Women of Advanced Maternal Age with Poor Embryo Survival: A Case Report. Gynecol Reprod Health. 2018; 2(2): 1-3.
Abstract
A crucial component of In-Vitro Fertilization (IVF) success is selection of excellent quality embryos for uterine transfer. The ability to correlate excellent quality embryos with successful implantation is diminished in women of advanced maternal age (AMA). In recent years, controversy has surrounded the universal use of preimplantation genetic screening (PGS) in women undergoing IVF. A 39-year-old Caucasian nulligravida with age related infertility underwent an unsuccessful fresh IVF cycle that was followed by the unusually poor survival of morphologically good quality frozen blastocysts. Subsequently, she underwent a second IVF cycle with PGS of fresh blastocysts as well as blastocysts from the first IVF cycle. A successful transfer of euploid embryos resulted in a live birth. This case, although not conclusive, delineates the additional benefit of using PGS in women of advanced maternal age with poor survival of morphologically good quality embryos.
Keywords
Introduction
Selection of excellent quality embryos for uterine transfer is critical to successful In-Vitro Fertilization (IVF) outcomes [1,2]. Morphologic characteristics of developing embryos have historically served as a determinant of embryo quality [3]. However, these characteristics are not predictive of chromosomal aberrations in embryos. Aneuploidy of embryos is a leading cause of IVF failure through both disruption of implantation and early miscarriage [4]. In recent years, technological advancements such as preimplantation genetic screening (PGS) has led to the selection of euploid embryos for transfer. These developments have improved the IVF live birth rates and decreased the risk of multiple gestations, overall improving IVF pregnancy outcomes [4-7]. Advanced maternal age (AMA) is defined as an age of 35 years or older at time of delivery [8]. AMA is a known indication for PGS in women undergoing IVF [9]. However, universal utilization of PGS in these patients is still an area of controversy despite data that is suggestive of the time and cost effectiveness of PGS in women of AMA [10,11]. Our case illustrates a benefit of PGS when poor survival of frozen blastocysts is encountered in otherwise morphologically normal embryos resulting from a non- PGS IVF cycle.
Case Description
A 39-year-old Caucasian nulligravida presented with age related infertility. Antral follicular count was 11 at her baseline ovarian reserve evaluation. Uterine assessment demonstrated an unicornuate uterus without a rudimentary horn. Anti-Mullerian Hormone (AMH) was 4.9 ng/mL. Her medical history was remarkable for Hashimoto’s thyroiditis with a documented.
Thyroid Peroxidase Antibody of 564 IU/mL. Her thyroid function was followed closely, and prior to fertility treatment she was on Levothyroxine 88mcg daily for repletion. Additionally, her gynecologic history was remarkable for a loop electrosurgical excision procedure of the cervix with normal cervical cytology for 10 years following the procedure. After three unsuccessful attempts of ovulation induction with clomiphene citrate 50mg and intrauterine insemination, she underwent IVF treatment.
Her first IVF cycle consisted of ovarian stimulation using Leuprolide acetate microdose protocol with recombinant Follitropin Alfa 225 IU and purified Menotrophin injectable 75 IU for a total of 10 days. A subcutaneous (SQ) trigger injection of 250 mg recombinant Human Chorionic Gonadotropin (r-HCG) was then administered and followed by oocyte retrieval 36 hours later. A single, fresh day 5 blastocyst of morphological grade 4AA- was transferred without any difficulties resulting in no conception. The first, fresh IVF cycle yielded 8 good quality, frozen blastocysts as reflected in Table 1. Grading of blastocysts was defined by the Gardner system. On day 5, there were a total of 5 blastocysts vitrified labeled #1-5 (4AA-, 5A-A-, 3.5AA-, 3AA, 2.5AA). Day 6 yielded an additional 3 blastocysts labeled #6-8 (4AA, 4AA-, 3A-A).
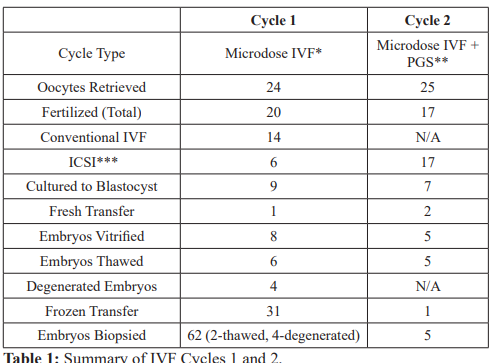
*In-Vitro Fertilization
**Preimplantation Genetic Screening
***Intracytoplasmic sperm injection.
- Two embryos were transferred prior to PGS and 1 was transferred following
- Four of the embryos were the degenerated blastocyst from initial thawing and 2 were the remaining 2 frozen.
Our patient then underwent a frozen embryo transfer preparation. She was administered oral micronized estrogen for 14 days as well as progesterone in oil 50 mg IM injections for 5 days once her endometrial stripe measured 9.5 mm. Six of the 8 blastocysts were thawed with surprising degeneration of four blastocysts. Thawing order was: embryos #1-2 (degenerated); #6-7 (#6 degenerated and
#7 4AA- was transferred); #3 and 8(#3 degenerated and #8 3A-A was transferred). Two surviving blastocysts #7 and #8 (4AA- and 3A-A) were easily transferred resulting in no conception.
The second IVF cycle consisted of ovarian stimulation using the same protocol and doses of gonadotropins as in the first IVF cycle with a total of 11 days of stimulation. A r-HCG trigger injection of 250 mg SQ was administered and followed by oocyte retrieval 36 hours later with cycle outcomes summarized in Table 1. Five, fresh blastocysts labeled #1-5 (4AA-, 4BA, 5AA, 3BA, 3BB-) from this cycle underwent trophectoderm biopsy and vitrification on day 6 with results as labeled in Table 2. The 2 remaining frozen embryos from the first IVF cycle were thawed before undergoing trophectoderm biopsy and vitrification. These embryos are labeled #6-7 (3AA, 2.5 AA) in Table 2. Given the unusual degeneration of 4 of 6 embryos from the first cycle, the decision was made to biopsy the degenerated embryos from the first IVF cycle, labeled #8-11 in Table 2. All biopsy samples were screened for chromosomal derangements using next generation sequencing (NGS) prior to being discarded.
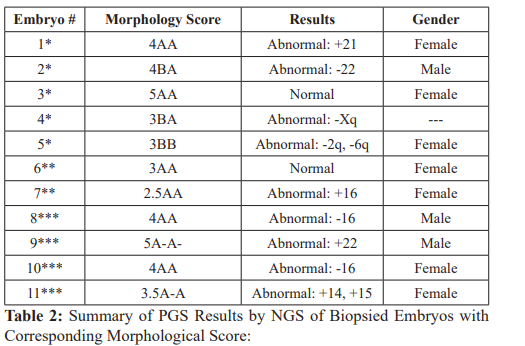
*Blastocysts from second IVF cycle fresh blastocyst biopsy.
**Thawed embryos from first IVF cycle with re-vitrification.
***Degenerated embryos from first IVF cycle discarded after biopsy.
The patient underwent our standard frozen embryo transfer preparation as previously described above. Transfer of the euploid blastocysts #3(5AA) from IVF cycle 2 and #6 (3AA) from IVF cycle 1 was performed and resulted in successful conception. A viable singleton pregnancy was confirmed by 8 week ultrasound. Amniocentesis at 20 weeks confirmed a normal 46XX karyotype. A healthy, female newborn was delivered at 35 weeks gestation following onset of preterm labor via cesarean section due to fetal malpresentation.
Discussion
We provide an example case in which preimplantation genetic screening was helpful in elucidating the etiology of poor survival of morphologically good quality embryos as well as in the selection of euploid embryos of transfer in a woman of advanced maternal age. Our patient’s first cycle was characterized by the surprising degeneration of 4 out of 6 good quality blastocysts. This was unusual for our lab as it is an early adopter of blastocyst cryopreservation and has consistently demonstrated survival rates of 96-98% over the last several years. In fact, we had several patients with successful outcomes on the same day our patient’s embryos were processed using the same laboratory technicians, media, and thawing processes.
Although not standard practice, due to patient request the 4 degenerated blastocysts from cycle 1 were also biopsied to evaluate chromosomal aberrations as the cause of embryo degeneration. PGS was performed on a total of 11 good quality blastocysts including 5 fresh embryos from the second cycle, 2 thawed embryos from the first cycle, and the 4 degenerated embryos from the first cycle. Aneuploidy was observed in 9 of the 11 biopsied embryos. Notably, all 4 of the degenerated blastocysts were aneuploid. These results are consistent with the known linear relationship between advanced maternal age and aneuploidy of embryos [5,12].
While the linear relationship between aneuploidy and maternal age is not novel, the universal use of PGS in women in their late thirties is controversial. PGS has limitations that thwart its applicability to all IVF protocols including: infrequent misdiagnosis resulting from mosaicism, sampling of limited cells, financial constraints, and lack of standard laboratory protocols for PGS [12,13]. However, PGS can shorten time to live births, minimize multiple gestations, and decrease miscarriage rates through the reduction of embryo aneuploidy [12,14,15]. Recent studies also support the cost effectiveness of PGS in women of AMA [11]. Accordingly, PGS may certainly have a place in IVF protocols of women of advanced maternal age. Our case illustrates that embryo genetic screening was helpful in decreasing the number of transfers needed to reach a live birth and provided a much-desired explanation to our patient and embryologists regarding the unusually poor survival of morphologically good quality embryos from initial non-PGS IVF cycle.
Conclusion
We present the case of a woman of advanced maternal age with poor survival of excellent quality frozen blastocysts associated with aneuploidy as demonstrated by preimplantation genetic screening. Although a single case report is insufficient to establish the need for PGS in all women of AMA, our case suggests a role for it within a certain subset of this population.
References
- Barash OO, Ivani KA, Willman SP, et al. Impact of Embryo Morphology on Clinical Pregnancy Rates in IVF PGS Cycles With Single Embryo Fertil Steril. 2017; 107: 18-19.
- Brown K, Thompson SM, Onwubalili N, et al. Blastocyst Expansion Score and Trophectoderm Morphology Strongly Predict Successful Clinical Pregnancy and Life Birth Following Elective Single Embryo Blastocyst Transfer (eSET): A National Study. J Assist Repro Genetics. 2013; 30:1577-1581.
- Rocha JC, Passalia F, Alves MF, et al. Methods for Assessing the Quality of Mammalian Embryos: How Far We Are From the Gold Standard?. JBRA Assist 2016; 20: 150-158.
- Forman EJ, Tao X, Ferry KM, et al. Single embryo transfer with comprehensive chromosome screening results in improved ongoing pregnancy rates and decreased miscarriage Hum Reprod. 2012; 27: 1217-1222.
- Lee HL, McCulloh DH, Hodes-Wertz B, et In vitro fertilization with preimplantation genetic screening improves implantation and live birth in women age 40 through 43. J Assist Reprod Genet. 2015; 32: 435-444.
- Rubio C, Bellver J, Rodrigo L, et al. Preimplantation genetic screening using fluorescence in situ hybridization in patients with repetitive implantation failure and advanced maternal age: two randomized Fertil Steril. 2013; 99: 1400-1407.
- William B Schoolcraft, Nathan R Treff, John M Stevens, et Live birth outcome with trophectoderm biopsy, blastocyst vitrification, and single-nucleotide polymorphism microarray- based comprehensive chromosome screening in infertile patients. Fertil Steril. 2011; 96: 638-640.
- Society for Maternal-Fetal Advanced Maternal Age and Risk of Antepartum Stillbirth. Contemporary OBGYN. 2012; 57: 22-26.
- Deveroey P, Staessen C, Devroey P, et Current Value of Preimplantation Genetic Screening in IVF. Hum Repro Update. 2007; 13: 15-25.
- Chen HF, Chen SU, Ma GC, et al. Preimplanatation Genetic Diagnosis and Screening: Current Status and Future J of the Formosa Medical Association. 2017; 116: 1-7.
- Collins SC, Xu X, Mak Cost Effectiveness of Preimplantation Genetic Screening for Women Older than 37 Undergoing In Vitro Fertilization. Obstetrics and Gynecology. 2017; 129: 168-169.
- Demko ZP, Simon AL, McCoy RC, et al. Effects of Maternal Age on Euploidy Rates in a Large Cohort of Embryos Analyzed with 24-Chromosome Single-Nucleotide Polymorphism- based Preimplantation Genetic Screening. Fertil Steril. 2016; 105: 1307-1313.
- Vendrell X, Carrero R, Alberola T, et al. Quality Management System in PGD/PGS: Now is the Time. J Assisted Reprod 2009; 26: 197-204.
- Brezina PR, Raymond WKe, William H Preimplantation Genetic Screening: A Practical Guide. Clin Med Insights Reprod Health. 2013; 7: 37-42.
- Capalbo A, Rienzi L, Cimadomo D, et Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod. 2014; 29: 1173-1181.